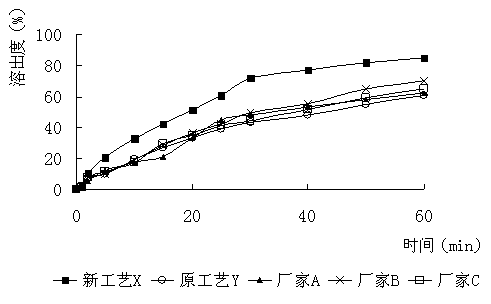
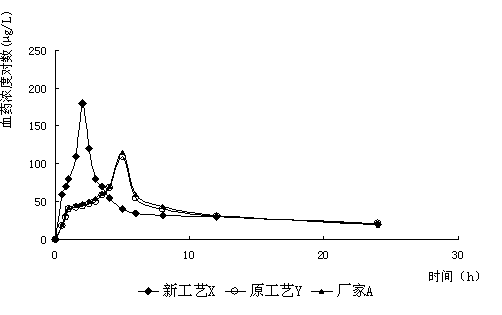
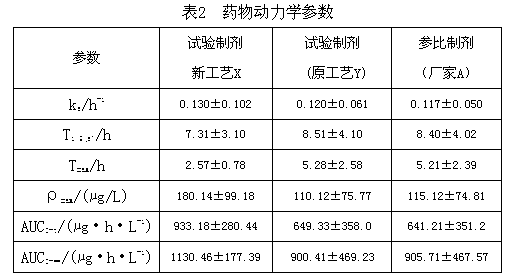
Furazolidone tablet preparation method
A furazolidone tablet and furazolidone technology are applied in the field of medicine to achieve the effects of convenient operation, improved bioavailability, and high dissolution rate in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Get furazolidone, copovidone, croscarmellose sodium and sieve 80 mesh, and silicon dioxide sieve 120 mesh. Dissolve 2.2 g of povidone and 1 g of propylene glycol in water to prepare a 1% adhesive solution. Mix 4 g of copovidone and 1.8 g of croscarmellose sodium evenly, and then mix evenly with 100 g of furazolidone in an equal amount incremental method. Add the aforementioned binder solution to make a soft material, granulate with 30 mesh, dry at 65°C, granulate with 30 mesh, add 1.0 g of silicon dioxide, mix evenly, and press into tablets according to the weight of 0.110 g. Disintegrate for 5 minutes, and dissolve 88%.
Embodiment 2
[0026] Furazolidone, dextrin, hydroxypropyl cellulose and povidone were sieved to 80 mesh, and silicon dioxide, magnesium stearate and talcum powder were sieved to 120 mesh. Dissolve 14g of starch, 0.8g of macrogol glyceride laurate, and 3g of sodium lauryl sulfate in hot water to prepare a 20% binder solution. Mix 3.2 g of dextrin, 4 g of hydroxypropyl cellulose, and 4 g of povidone evenly, and then mix evenly with 100 g of furazolidone by equal addition method. Add the aforementioned binder solution to make soft material, granulate with 16 mesh, granulate with 18 mesh after drying at 70°C, add 2g of silicon dioxide, 2g of magnesium stearate and 2g of talc powder, mix evenly, and press the tablet according to the weight of 0.135g. have to. It disintegrates for 5 minutes and dissolves 82%.
Embodiment 3
[0028] Furazolidone, hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone and cyclodextrin were sieved to 80 mesh, and magnesium stearate and silicon dioxide were sieved to 120 mesh. Dissolve 10 g of hydroxypropyl methylcellulose and 3 g of sodium lauryl sulfate in water to prepare a 2% binder solution. Mix 8 g of hydroxypropyl cellulose, 10 g of cross-linked polyvinylpyrrolidone, and 10 g of cyclodextrin evenly, and then mix evenly with 100 g of furazolidone by the method of equal addition. Add the aforementioned binder solution to make a soft material, granulate with 12 mesh, dry at 70°C and granulate with 30 mesh, add 2.0g of magnesium stearate and 2.0g of silicon dioxide, mix evenly, and press into tablets according to the weight of 0.145g. Disintegrate for 5 minutes, and dissolve 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com