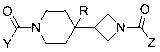
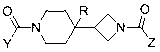
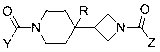
Piperidin-4-yl-zetidine diamides as monoacylglycerol lipase inhibitors
A pyridyl and phenyl technology, applied in the directions of anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve problems such as difficulty in separating side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0324]
[0325] A. tert-Butyl 3-(pyridin-4-yl)azetidine-1-carboxylate, 1c. equipped with thermocouple, magnetic stirrer, condenser, heating mantle and N 2A 1-liter 3-neck round bottom flask with an inlet adapter was charged with anhydrous dimethylacetamide (DMA, 100 mL) and zinc (42.94 g, 650.2 mmol). The mixture was stirred at 20 °C while 1,2-dibromoethane (DBE, 5.38 mL, 62.34 mmol) and trimethylchlorosilane (TMS-Cl, 7.54 mL, 59.28 mmol). The resulting slurry was aged for 15 min. A solution of tert-butyl 3-iodoazetidine-1-carboxylate 1a (122.78 g, 420.69 mmol) in DMA (201 mL) was added dropwise at a rate to keep the temperature below 65 °C over 1 h, and the milky The suspension was stirred for 30 min while cooling slowly to 20 °C.
[0326] in N 2 Under the conditions equipped with thermocouple, mechanical stirrer, condenser, heating mantle and N 2 [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane complex (4.73 g, 5.74 mmol), cuprous iodide ...
example 2
[0337]
[0338] A. 3-Chloro-6-fluorobenzo[b]thiophene-2-carbonyl chloride, 2b. Thionyl chloride (73.7 mmol, 5.36 mL) was added to a mixture of 4-fluorocinnamic acid 2a (21.1 mmol, 3.5 g) and pyridine (2.53 mmol, 0.2 mL). The mixture was heated at 135 °C for 30 min, then cooled to room temperature. The crude mixture was triturated with hot hexanes to remove the solid pyridine hydrochloride by-product. Compound 2b was isolated from the combined hexane solutions.
[0339]B. 4-{1-[(3-chloro-6-fluoro-1-benzothiophen-2-yl)carbonyl]azetidin-3-yl}-1-(1,3-thiazole-2 -ylcarbonyl)piperidine, Cpd8. Compound 2b (0.45mmol, 112mg) was dissolved in 4mL CH at 0°C 2 Cl 2 The solution in was added compound 1j mono-TFA salt (0.41mmol, 150mg) in Et 3 N (2.46mmol, 0.34mL) in solution. The resulting reaction mixture was stirred at 0 °C for 3 h. The crude product was purified by preparative reverse phase chromatography to provide 18 mg (9% yield) of Cpd8. 1 H NMR (CD 3 OD, 400MHz): δ=7.8...
example 3
[0343]
[0344] A. 3-Methyl-6-(trifluoromethyl)benzo[b]thiophene-2-carboxylate, 3c. Methyl thioglycolate 3b (30.3 mmol, 2.76 mL) was added dropwise to a suspension of NaH (60% oil dispersion, 75.8 mmol, 3.03 g) in 10 mL THF and 50 mL DMSO at 20 °C. The mixture was stirred for 15 min, and a solution of 1-(2-fluoro-4-(trifluoromethyl)phenyl)ethanone 3a (24.3 mmol, 5.0 g) in 10 mL DMSO was added. The reaction mixture was stirred at 20 °C for 4 h, and water was added. The mixture was extracted with EtOAc. MgSO for organic layer 4 Drying and concentration afforded compound 3c as a white solid.
[0345] B.4-(1-{[3-methyl-6-(trifluoromethyl)-1-benzothiophen-2-yl]carbonyl}azetidin-3-yl)-1-(1 , 3-thiazol-2-ylcarbonyl)piperidine, Cpd20. To compound 1j mono-TFA salt (0.27mmol, 100mg) and compound 3c (0.30mmol, 78mg) in 4mL CH 2 Cl 2 Et was added to the stirred solution in 3 N (1.09 mmol, 0.15 mL). After 20 min at 20 °C, HATU (0.33 mmol, 125 mg) was added and the mixture was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com