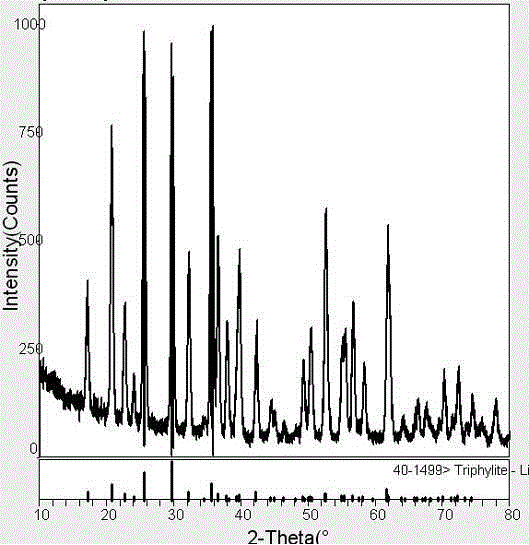
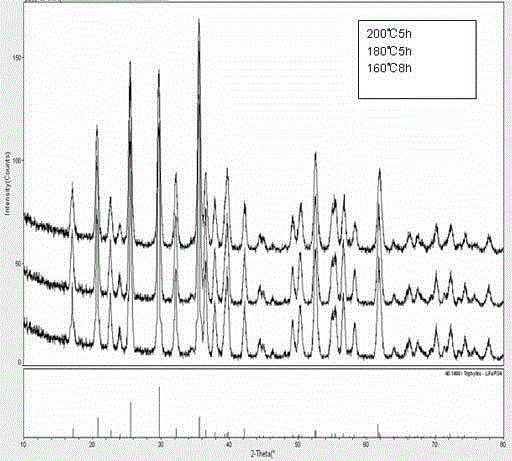
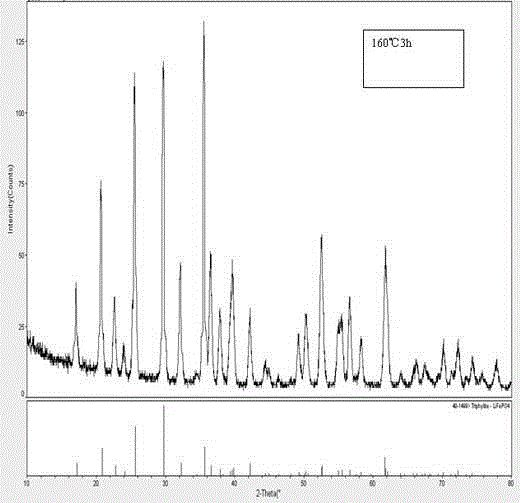
Method for preparing lithium iron phosphate cathode material by hydrothermal method
A technology of lithium iron phosphate and positive electrode materials, which is applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problems of uneven particle size, wide range of particle size distribution, and high reaction temperature, so as to reduce the reaction temperature and improve Magnification performance, good mutual contact effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of LiFePO by Hydrothermal Method 4 The method comprises the following steps in turn:
[0024] (1) First weigh 0.3mol Li0H and add deionized water to dissolve it in a 500ml beaker, then add 0.1molH 3 P0 4 Slowly add LiOH solution along the wall of the vessel, and centrifuge to obtain Li 3 P0 4 White precipitate, dried under vacuum at 50°C-60°C to obtain LiFePO 4 powder.
[0025] (2) Add in another beaker, and weigh Li respectively at a molar ratio of 1:3 3 PO 4 = 0.5891g; and (NH 4 ) 2 Fe(SO 4 ) 2 =1.9877g, M (glucose)=0.1660g is added deionized water and is dissolved in the beaker, after stirring evenly, add to the stainless steel reaction kettle, the filling amount of described mixed solution in the reaction kettle is 60%.
[0026] (3) Put it into the reaction kettle and heat it to 200°C, heat for 5 hours, cool to room temperature after the reaction, and take out the reaction kettle; use deionized water and detergent to separate the sediment thre...
Embodiment 2
[0028] (1) First weigh 0.3mol Li0H and add deionized water to dissolve it in a 500ml beaker, then add 0.1molH 3 P0 4 Slowly add LiOH solution along the wall of the vessel, and centrifuge to obtain Li 3 P0 4 White precipitate, dried under vacuum at 50°C-60°C to obtain LiFePO 4 powder.
[0029] (2) Add in another beaker, and weigh Li respectively at a molar ratio of 1:3 3 PO 4 = 0.5882g; and (NH 4 ) 2 Fe(SO 4 ) 2 =1.9802g, M (glucose)=0.1607g add deionized water and dissolve in the beaker, stir and add to the stainless steel reactor after stirring, the filling amount of the mixed solution in the reactor is 60%.
[0030] (3) Put it into the reaction kettle and heat it to 180°C, heat it for 5 hours, cool to room temperature after the reaction is over, take out the reaction kettle; use deionized water and lotion to separate the sediment three times, and then use ethanol lotion for three times after centrifugation , dried under vacuum at 60°C to obtain LiFePO 4 powder
Embodiment 3
[0032] Preparation of LiFePO by Hydrothermal Method 4 The method comprises the following steps in turn:
[0033] (1) First weigh 0.3mol Li0H and add deionized water to dissolve it in a 500ml beaker, then add 0.1molH 3 P0 4 Slowly add LiOH solution along the wall of the vessel, and centrifuge to obtain Li 3 P0 4 White precipitate, dried under vacuum at 50°C-60°C to obtain LiFePO 4 powder.
[0034] (2) Add in another beaker, and weigh Li respectively at a molar ratio of 1:3 3 PO 4 = 0.3671g; and (NH 4 ) 2 Fe(SO 4 ) 2 =1.2464g, M (glucose)=0.0848g is added deionized water and is dissolved in the beaker, is added to the stainless steel reactor after stirring evenly, and the filling amount of described mixed solution in the reactor is 60%.
[0035] (3) Put it into the reaction kettle and heat it to 160°C, heat it for 8 hours, cool to room temperature after the reaction, and take out the reaction kettle; use deionized water and detergent to separate the sediment three tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com