Pharmaceutical preparation for rapidly releasing citric acid benzene ring tinib and preparation method thereof
A technology for cyclobenztinib citrate and a pharmaceutical preparation, which is applied in the field of rapid-release drug cyclobentinib tablets and the preparation thereof, can solve the problems such as the inability to meet the treatment requirements of different types of patients, and achieves shortening the granulation time, No serious toxicity occurs, the effect of avoiding the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 cyclobenztinib citrate and pharmacodynamic research result
[0040] (1) Preparation and structure confirmation of cyclobenztinib citrate
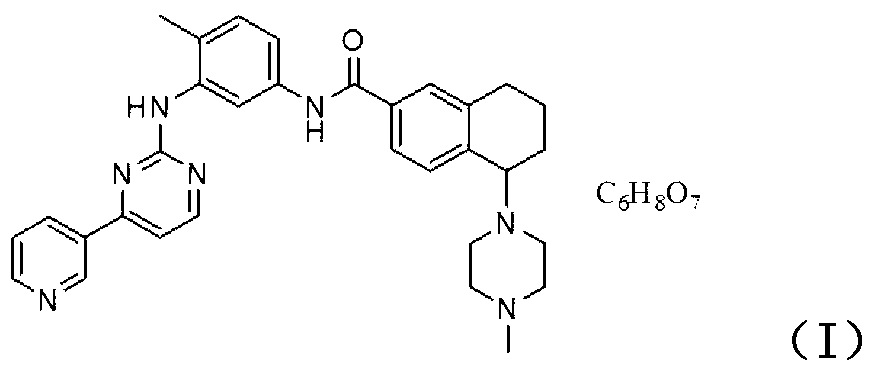
[0041] Add N-{4-methyl-3-[(4-pyridin-3-yl)pyrimidine-2-amino]phenyl}-5-(4-methylpiperazin-1-yl)-5 to the reaction flask, 6,7,8-tetrahydronaphthalene-2-amide (5.33 g, 10 mmol), 200 ml of absolute ethanol, citric acid (1.01 g, 10.5 mmol), stirred and refluxed at 70°C for 0.5 hours, After cooling to room temperature, a solid precipitated out, which was filtered out by suction and recrystallized by stirring with 180 ml of ethanol. Filter and dry to get the product N-{4-methyl-3-[(4-pyridin-3 base)pyrimidine-2-amino]phenyl}-5-(4-methylpiperazin-1-yl)- 5.94 g of 5,6,7,8-tetrahydronaphthalene-2-amide citrate, namely cyclobenztinib citrate, with a yield of 94.5%.
[0042] Molecular formula: C 32 h 35 N 7 O·C 6 h 8 o 7 .
[0043] Melting point: 191-193°C.
[0044] 1 H-NMR (DMSO-d 6 ppm): δ9.98(s,1H...
Embodiment 5
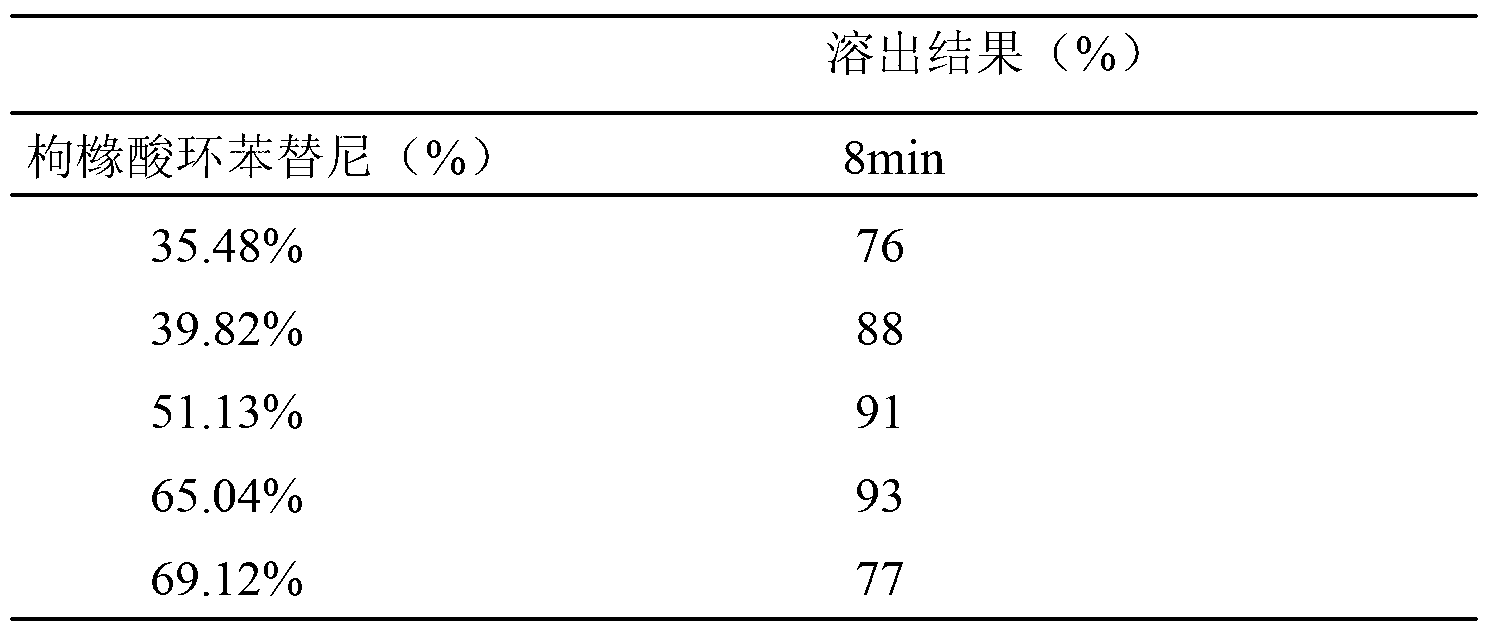
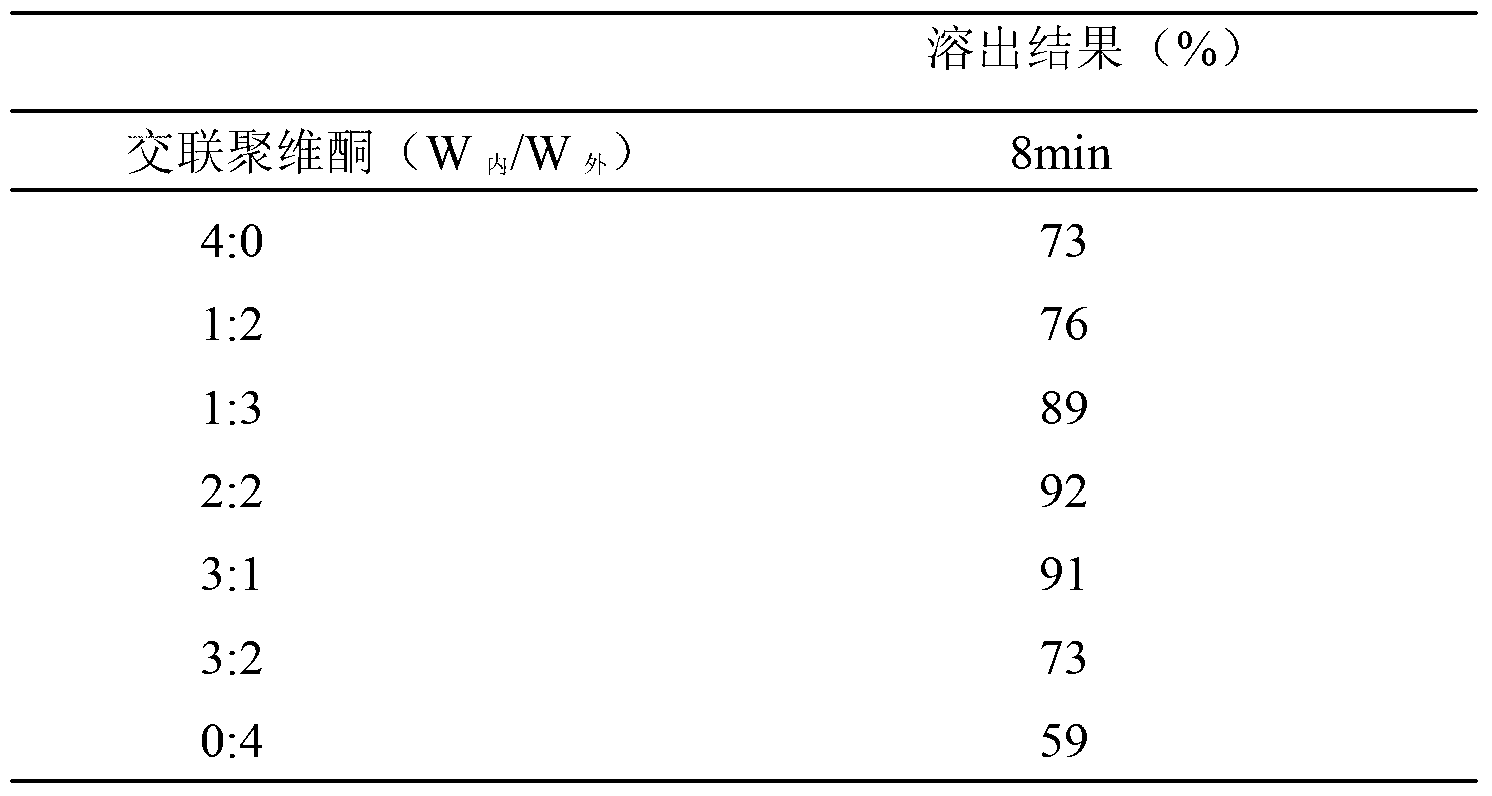
[0065] Embodiment 5 rapid release cyclobenztinib citrate tablet
[0066] (1) The formula is as follows:
[0067]
[0068]
[0069] (2) The preparation method is as follows:
[0070] 1) Pass cyclobenztinib citrate through a 200-mesh sieve;
[0071] 2) Weigh cyclobenztinib citrate, microcrystalline cellulose and crospovidone, and mix them evenly;
[0072] 3) Weigh the added part of talc powder and polyethylene glycol 6000, mix well;
[0073] 4) Dry granulation machine granulation, pressure 5MPa;
[0074] 5) Add the additional part of crospovidone, talcum powder and polyethylene glycol 6000 according to the prescription ratio, and mix well;
[0075] 6) Compressed into tablets, hardness 6-8Kg / cm 2 .
Embodiment 6
[0077] The tablet obtained in Example 5 was coated with Opadry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com