Medium freeze-drying powder production process for lymphocyte culture
A production process and technology of lymphocytes, applied in the field of medium freeze-dried powder production process, can solve the problems such as no medium freeze-drying process or method has been found, and achieve the goal of improving activity and stability, stable effect and reducing production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Freeze-dried culture medium of the present invention and traditional liquid culture medium activity, stability study result comparison
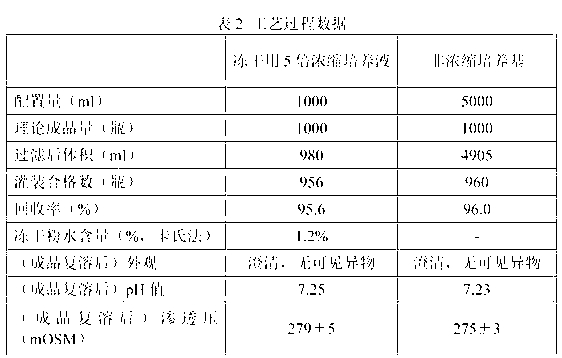
[0035] 1. Prepare respectively the 5-fold concentrated culture solution and the traditional liquid medium for freeze-drying of the present invention in the order of the formula in Table 1. Among them, RPMI1640 dry powder is from GIBCO Company, and newborn calf serum is from Hangzhou Sijiqing Company. After each material was added, stir with a magnetic stirrer at 300 rpm for two hours at room temperature to dissolve.
[0036]
[0037] 2. Filter the two solutions separately: the dissolved solution is first clarified and pre-filtered with a filter with a pore size of 1 um, and then sterile-filtered with a filter with a pore size of 0.22 um. The filter used is a 5-inch cartridge filter, and the corresponding 1 um and 0.22 um filter elements are all made of PES; the filtration flow rate is set to 1 L / hr. The filtration steps for non-co...
Embodiment 2
[0065] Comparison of the effects of lymphocyte culture medium freeze-dried powder prepared by different formulas or freeze-drying processes
[0066] See Table 4 for the differences and culture results between the freeze-dried lymphocyte medium process of the present invention and other freeze-dried medium processes. Wherein the concrete process parameter of process 1-3 is the same as embodiment 1, only the ratio of nitrogen and oxygen is different; The gas that process 4 feeds is common air, and other process parameters are with embodiment 1; Process 5 is due to the needs of dissolving mannitol, needs The filling volume is added, so the process parameters of sublimation and secondary drying are adjusted accordingly, and the gas introduced is ordinary air.
[0067] It can be seen from Table 4 that the osmotic pressure value of the lyophilized powder reconstituted solution prepared by adding mannitol in process 5 has exceeded the upper limit of normal lymphocyte culture (290mOSM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com