Rosin or rosin derivative long chain flexible monomer preparation method
A technology of rosin derivatives and flexible monomers, which is applied in the field of preparation of rosin or rosin derivatives long-chain flexible monomers, achieving the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The first step is to dissolve rosin or rosin derivatives in dichloromethane or tetrahydrofuran, add an acid chloride reagent, and react at 0-85°C for 1-12 hours to synthesize rosin or rosin derivative acid chlorides; the rosin can be selected from gum rosin, Tall rosin and wood rosin. Rosin derivatives can be hydrogenated rosin, disproportionated rosin, acrylic rosin, maleic rosin, dehydroabietic acid, L-pimaric acid, maleopimaric acid, isopimaric acid; the acid chloride reagent can be oxalyl chloride (C 2 o 2 Cl 2 ), phosphorus trichloride (PCl 3 ), phosphorus pentachloride (PCl 5 ), thionyl chloride (SOCl 2 ), phosphorus oxychloride (POCl 3 ); the temperature can be selected from 0°C, 10°C, 20°C, 30°C, 40°C, 50°C, 60°C, 70°C, 80°C, 85°C. The reaction time can be selected from 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, 11 hours, 12 hours;
[0032] The second step is to mix rosin or rosin derivative acid chloride wit...
Embodiment 2
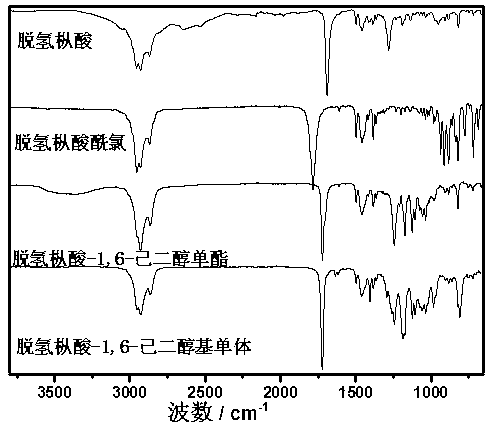
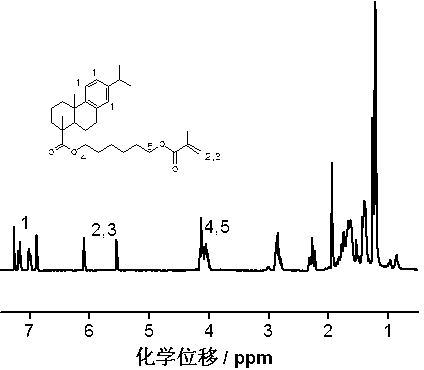
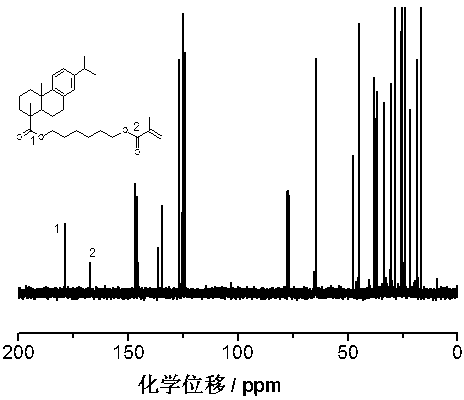
[0035] Synthesis of dehydroabietic acid-1,6-hexanediol-based long-chain flexible monomer
[0036] The synthetic route is shown below,
[0037]
[0038]In order to prepare flexible resin acid-based soft monomers, first connect hexanediol to dehydroabietic acid (DA), and use excess hexanediol (hexanediol / dehydroabietic acid=10 / 1 (molar ratio)) Dehydroabietic acid-1,6-hexanediol monoester can be efficiently synthesized with dehydroabietic acid chloride (DA-Cl), and then dehydroabietic acid-1 can be synthesized by reacting with a slight excess of methacryloyl chloride, 6-Hexanediol-based monomer;
[0039] The synthesis steps are:
[0040] (1) Add 1.00g of dehydroabietic acid (0.0033mol) into the reactor, dissolve it with 30ml of dichloromethane, add 0.6ml of oxalyl chloride (0.0055mol), and react at 25°C for 3 hours to obtain dehydroabietic acid chloride; ( 2) Add 4.10 g (0.033 mol) of catalyst 4-dimethylaminopyridine (DMAP) and 0.033 mol of 1,6-hexanediol to the reaction pr...
Embodiment 3
[0043] Synthesis of dehydroabietic acid-1,6-hexanediol-based long-chain flexible monomer
[0044] The synthetic route is shown below,
[0045]
[0046] The synthesis steps are:
[0047] (1) Add 1.00g of dehydroabietic acid (0.0033mol) into the reactor, dissolve it with 30ml of dichloromethane, add 0.6ml of oxalyl chloride (0.0055mol), and react at 25°C for 3 hours to obtain dehydroabietic acid chloride; ( 2) Add 4.10 g (0.033 mol) of catalyst 4-dimethylaminopyridine (DMAP) and 0.033 mol of 1,6-hexanediol to the reaction product of the first step, and react at 50° C. for 12 hours. The DMAP salt was filtered off, the resulting filtrate was dropped into petroleum ether and the corresponding precipitate was filtered off again, the filtrate was collected, and the solvent was removed to obtain 1.00 g of dehydroabietic acid-1,6-hexanediol monoester. (3) Add 0.40 g (1.0 mmol) of dehydroabietic acid-1,6-hexanediol monoester, 2.5 mmol of catalyst triethylamine (TEA) and 0.00010 g o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com