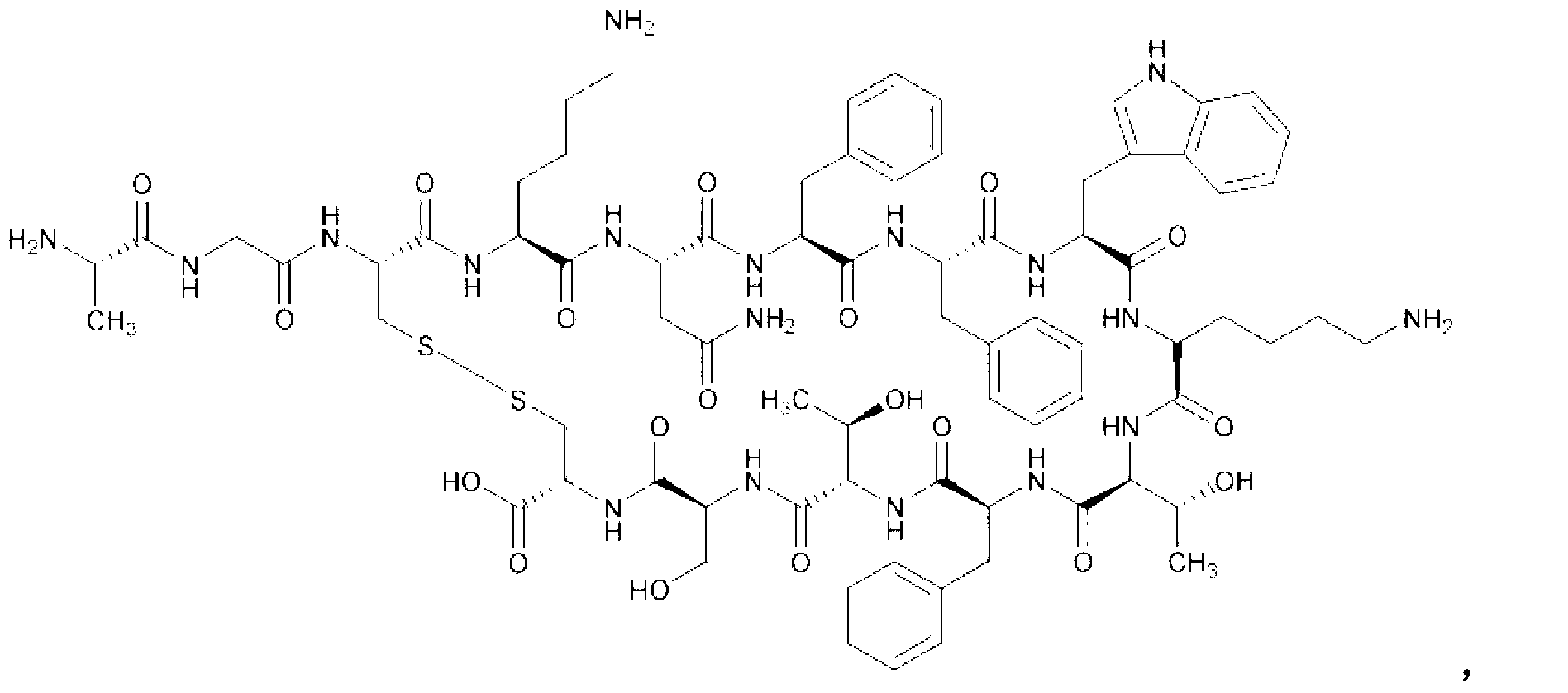
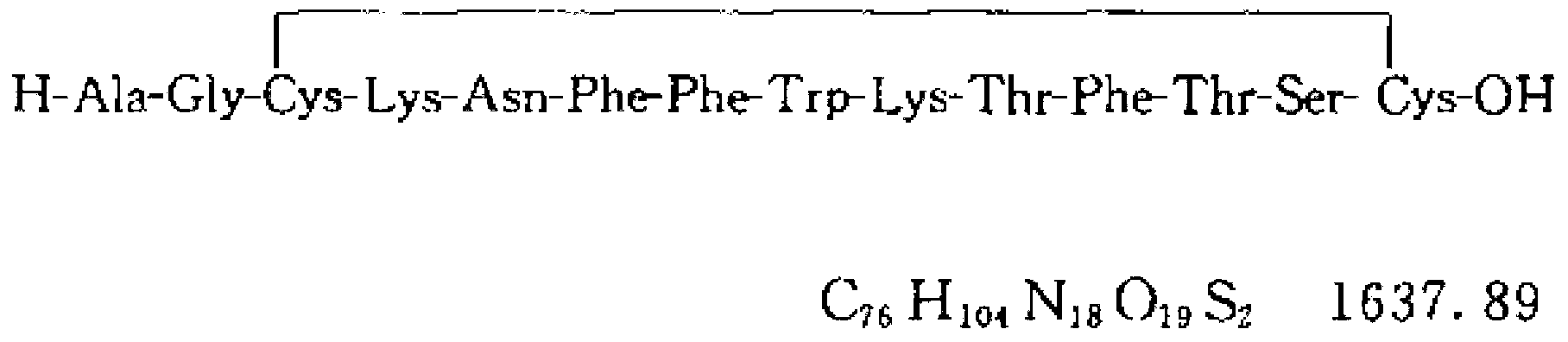Somatostatin freeze-dried powder injection
A technology for freeze-dried powder injection and somatostatin, which is applied in the field of medical technology and can solve problems such as insufficient stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0094] Preparation Example 1, Preparation of Powder Injection Containing Somatostatin
[0095] formula:
[0096] Somatostatin
0.75mg,
50mg,
5 mg,
pH regulator
to pH5.5,
Water for Injection
Appropriate amount, add to 1ml.
[0097] Preparation:
[0098] (1) Weigh the main drug and excipients (except pH adjuster) of the prescribed amount, place them in a stainless steel bucket, add about 80% of the prescribed amount of water for injection, dissolve each component, and then add 0.1% (w / v) of activated carbon, stirred for 30 minutes, filtered and decarbonized, and added water for injection to close to the full amount of the prescription.
[0099] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (the specified value is the value of the measured pH value of the freeze-dried dry powder diluted with water for injecti...
preparation example 2
[0104]Supplementary Preparation Example 2: Referring to the method of Preparation Example 1 above, the difference is that the amount of mannitol in it is adjusted to 0mg, 5mg, 10mg, 25mg, 75mg, 100mg, 150mg, and 200mg, and the numbers of the obtained powder injections are respectively Ex120, Ex121, Ex122, Ex123, Ex124, Ex125, Ex126, Ex127. For the case where the solid content is greater than 10%, add an appropriate amount of water to make the solid content 10%.
preparation example 3
[0105] Supplementary Preparation Example 3: Referring to the method of Preparation Example 1 above, the difference is that the mannitol in it is replaced by 25 mg of lactose, 75 mg of lactose, 250 mg of dextran, 75 mg of dextran, 25 mg of glycine, 75 mg of glycine, 25 mg of sodium chloride, Sodium chloride 75mg, the powder injection number that obtains is respectively Ex131, Ex132, Ex133, Ex134, Ex135, Ex136, Ex137, Ex138.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com