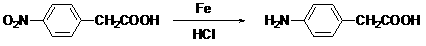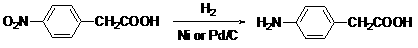Preparation method of p-aminophenylacetic acid
A technology of p-aminophenylacetic acid and p-nitrophenylacetic acid, applied in the field of preparation of p-aminophenylacetic acid, can solve problems such as higher requirements for reaction operating conditions, high preparation cost, and cost reduction, and achieves low cost and low production cost , the effect of good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Add 18g of p-nitrophenylacetic acid, 180g of ethanol (95%) and 0.36g of 3%Pd / attapulgite catalyst into the pressure reactor, replace the air in the reactor with nitrogen, and then pass in hydrogen to control 0.4MP and temperature 30-40 React at ℃ for 5 hours; let stand to cool to room temperature, open the pressure reactor, and evaporate 60% solvent ethanol; cool and crystallize, filter, and dry to obtain a crude product; recrystallize the crude product with 95% ethanol, decolorize with activated carbon, and obtain light white crystals 12.1g. The yield is 81%.
Embodiment 2
[0020] Example 2: Add 18g of p-nitrophenylacetic acid, 180g of ethanol (95%) and 0.54g of 4%Pd / attapulgite catalyst into the pressure reactor, replace the air in the reactor with nitrogen, and then pass in hydrogen to control 0.5MP and temperature 40-50 React at ℃ for 4 hours; let stand to cool to room temperature, open the pressure reactor, and evaporate 70% solvent ethanol; cool and crystallize, filter, and dry to obtain the crude product; recrystallize the crude product with 95% ethanol, decolorize with activated carbon, and obtain white crystal 12.4 g. The yield is 83%.
Embodiment 3
[0021] Example 3: Add 18g of p-nitrophenylacetic acid, 180g of ethanol (95%) and 0.72g of 5% Pd / attapulgite catalyst into the pressure reactor, replace the air in the reactor with nitrogen, and then pass in hydrogen, control 0.6MP, temperature 50-60 React at ℃ for 3 hours; let stand to cool to room temperature, open the pressure reactor, and evaporate 80% solvent ethanol; cool and crystallize, filter, and dry to obtain the crude product; recrystallize the crude product with 95% ethanol, decolorize with activated carbon, and obtain white crystal 14.2 g. The yield is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com