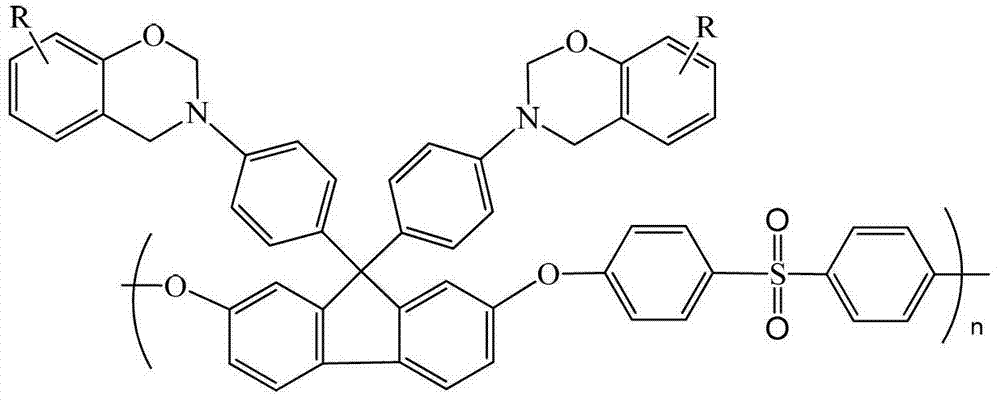
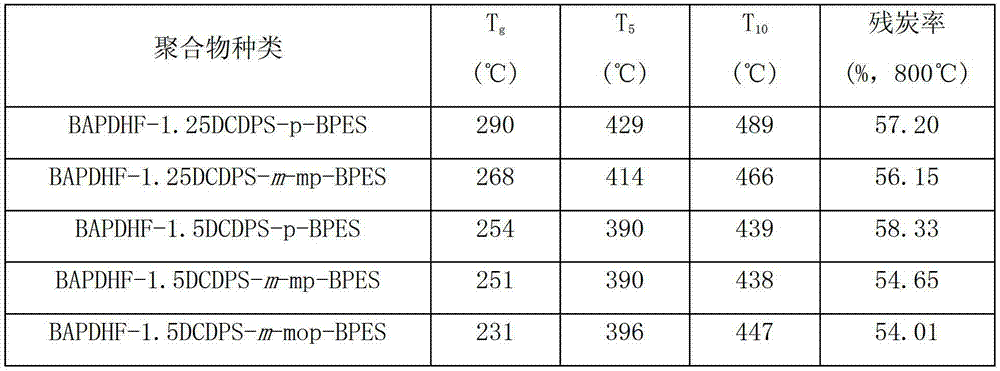
Fluorenyl polyether sulfone resin with side chains containing benzoxazine and preparation method of fluorenyl polyether sulfone
A technology of fluorenyl polyethersulfone and benzoxazine, which is applied in the field of thermoplastic resin and its preparation, can solve the problems of small molecular weight and brittleness of polymers, and achieve good thermal and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
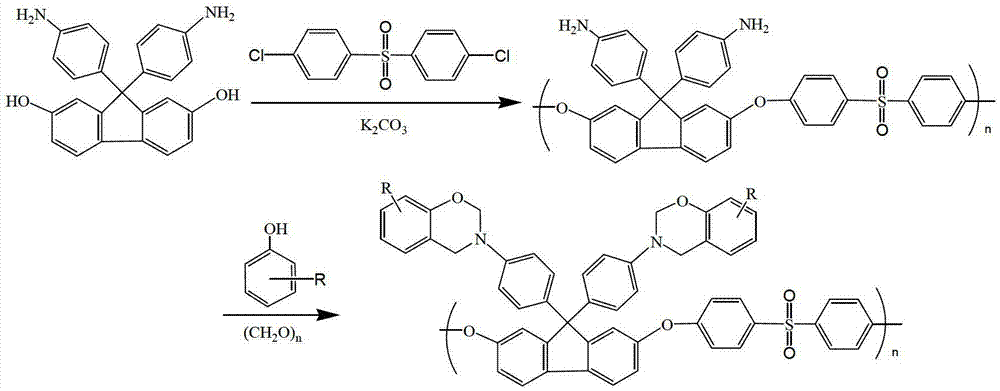
[0023] Synthesis of Fluorenyl Polyethersulfone with Amino Side Chain
[0024] Add 3.04g of 9,9-bis(4-aminophenyl)-2,7-dihydroxyfluorene, 1.48g of anhydrous potassium carbonate and Add 2.87g of dichlorodiphenyl sulfone, add 50mL dimethyl sulfoxide and 20mL toluene, blow nitrogen, raise the temperature to 160°C under stirring, after 3 hours of reaction, no more water will be brought out from the water separator, and the toluene will be evaporated. After continuing the reaction for 3 hours, the reaction was terminated, the reaction solution was cooled to room temperature, filtered, the filtrate was added to water, and the precipitate was precipitated, washed with water and ethanol, and finally vacuum-dried at 60°C for 24 hours to obtain a white fluorenyl group containing amino groups in the side chain The polyethersulfone powder was 4.78g, and the yield was 87%.
[0025] H NMR test results (500M, CDCl 3 , ppm): In the 1H NMR spectrum, δ=6.39~7.94ppm is the chemical shift of the...
Embodiment 2
[0027] Except that anhydrous potassium carbonate was changed to 1.65g and dichlorodiphenyl sulfone was changed to 3.44g, and the reaction temperature was changed to 180°C, other conditions were the same as in Example 1, and finally 4.16g of white side chain amino group-containing fluorenyl polyethersulfone powder was obtained. , yield 75%.
Embodiment 3
[0029] Synthesis of Fluorenyl Polyethersulfone Containing Benzoxazine Groups in Side Chains
[0030] Add 1.5g of fluorenyl polyethersulfone with amino groups in the side chains synthesized in Example 1, 0.94g of phenol, 30mL of xylene and 0.6g of paraformaldehyde into a three-necked flask in sequence, raise the temperature to 150°C, react for 6 hours, then cool down to room temperature, the reaction solution was poured into ethanol, filtered, the filter cake was washed with ethanol, and then vacuum-dried to obtain 1.2 g of fluorenyl polyethersulfone powder containing benzoxazine groups in the light yellow side chain, with a yield of 60 %.
[0031] H NMR test results (500M, CDCl 3 , ppm): In the 1H NMR spectrum, δ=6.77~7.81ppm is the chemical shift of the proton on the benzene ring, and 5.29ppm is the O-CH on the oxazine ring 2 The proton characteristic peak of -N, 4.69ppm is Ar-CH 2 The proton characteristic peak of -N. Infrared spectrum test results (KBr, cm -1 ): 3030cm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com