Sulfonamide compound and medicinal compositions thereof, and preparation methods and applications thereof
A compound, benzenesulfonamide-based technology, applied in the direction of drug combination, sulfonamide preparation, organic chemistry, etc., can solve problems such as the limitation of tumor therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
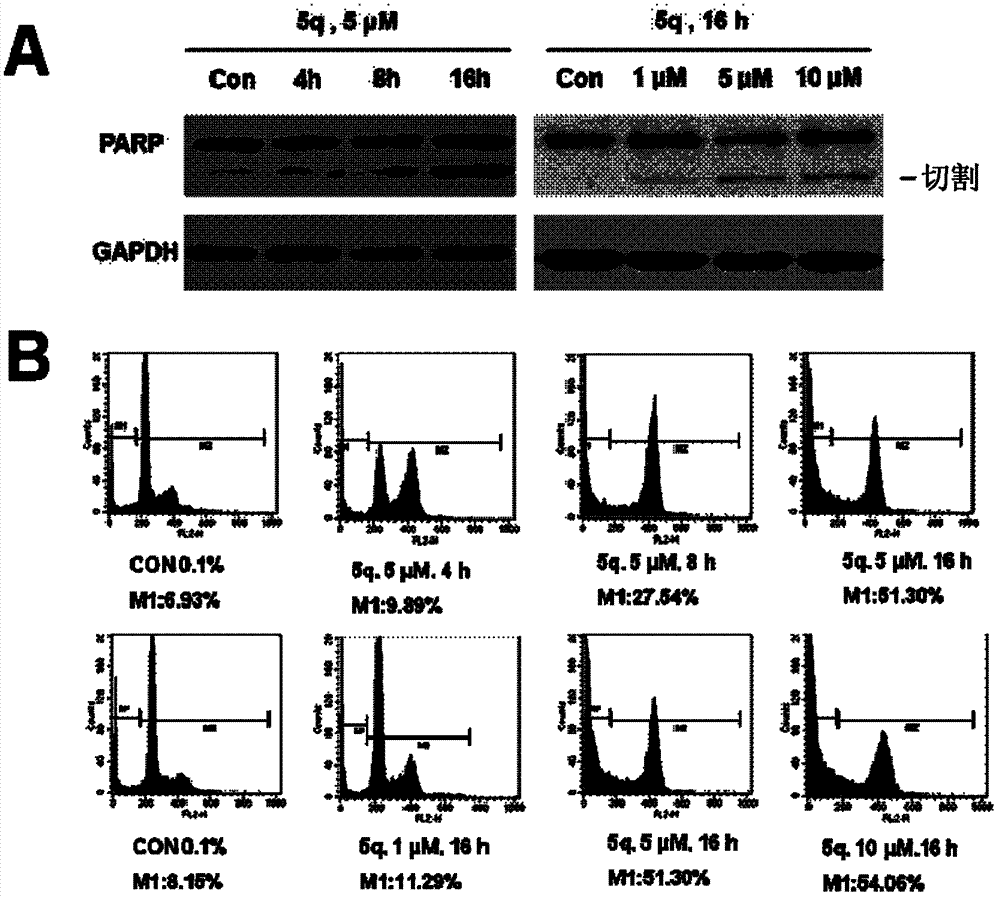
Image
Examples
preparation example Construction
[0152] Compound preparation
[0153] The compounds represented by general formula V and general formula X of the present invention can be prepared according to the following scheme and referring to synthetic methods known in the art. In general, in the preparation schemes described below, each reaction is carried out at -10°C to reflux temperature, usually at room temperature (about 25°C) to reflux temperature. Preferably, the reaction temperature is 5-100°C, more preferably 20-80°C. The reaction time is usually not particularly limited, and generally ranges from 1 minute to 24 hours, preferably from 1 to 20 hours. Wherein, the solvent used is usually a polar solvent, such as water, DMF, alcohol (such as methanol, ethanol, isopropanol, etc.). Synthesized compounds can be used physical and chemical methods, such as hydrogen spectrum ( 1 H-NMR), mass spectrometry (MS) and elemental analysis for structural identification.
[0154] A preparation method of a compound of formula...
Embodiment 1
[0194] Compound 4e: N-(4-aminophenyl)-2-(4-methyl-N-(3-(trifluoromethyl)phenyl)benzenesulfonamido)acetamide
[0195]
[0196] Step 1: 4-Methyl-N-(3-(trifluoromethyl)phenyl)benzenesulfonamide
[0197] Dissolve m-trifluoromethylaniline (1.61g, 10mmol), TsCl (1.91g, 10mmol), DMAP (1.34g, 11mmol) in dichloromethane (30ml), stir at room temperature for 30 minutes, then wash with saturated NaHCO 3 Wash (10ml*3), saturated NaCl wash (10ml*3), anhydrous NaCl 2 SO 4 Drying and column chromatography gave product a as a white solid (2.9 g, 92%). Mp = 80-82°C, 1 HNMR (CDCl 3 , 300MHz) δ: 2.39(s, 3H), 6.97(s, 1H), 7.24-7.37(m, 6H), 7.68(d, J=8.1Hz, 2H).
[0198] Step 2: tert-butyl 2-(4-methyl-N-(3-(trifluoromethyl)phenyl)benzenesulfonamido)acetate
[0199] Compound a (540mg, 1.7mmol) was dissolved in DMF (10ml), potassium carbonate (480mg, 3.4mmol) and tert-butyl bromoacetate (380μl, 2.6mmol) were added successively, stirred at room temperature for 2h, and then diluted with 50ml of ...
Embodiment 2
[0205] Compound 1a: 4-Methyl-N-(3-(trifluoromethyl)phenyl)benzenesulfonamide
[0206]
[0207] It was synthesized by the method shown in step 1 in Example 1.
[0208] white solid. 1 H NMR (300MHz, CDCl 3 )δ7.74(d, 2H), 7.38-7.21(m, 4H), 6.91(d, 2H), 3.83(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com