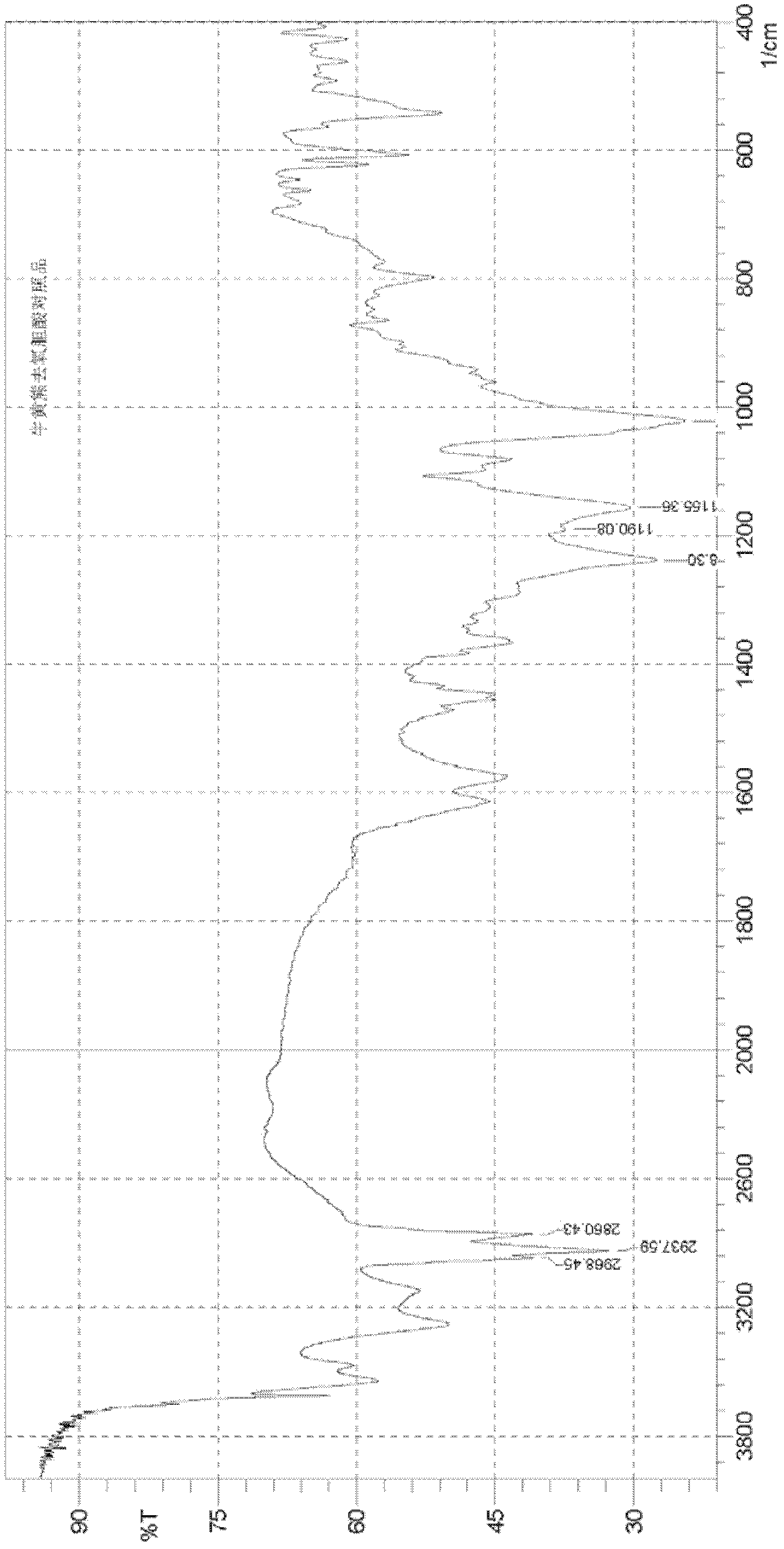
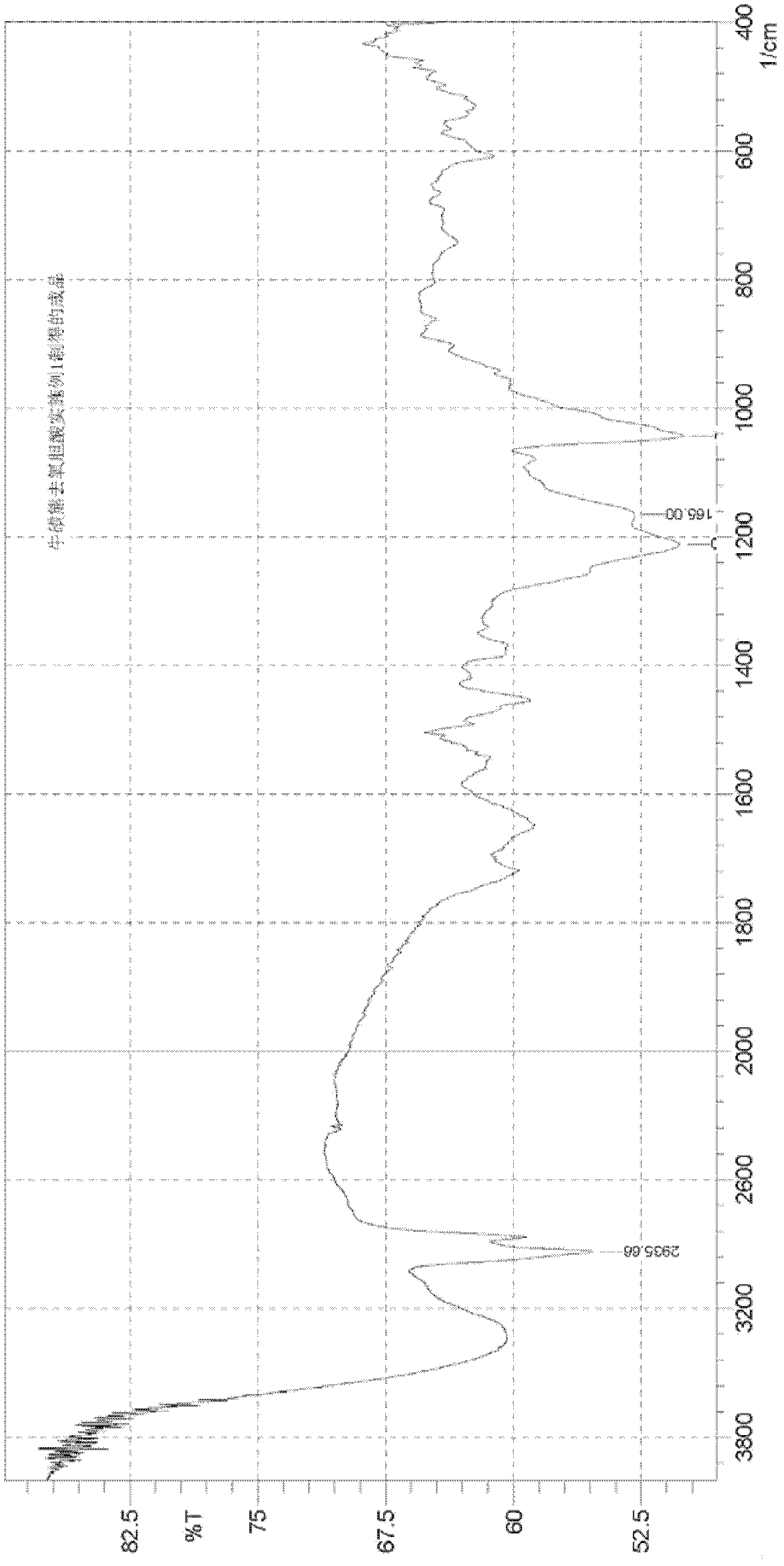
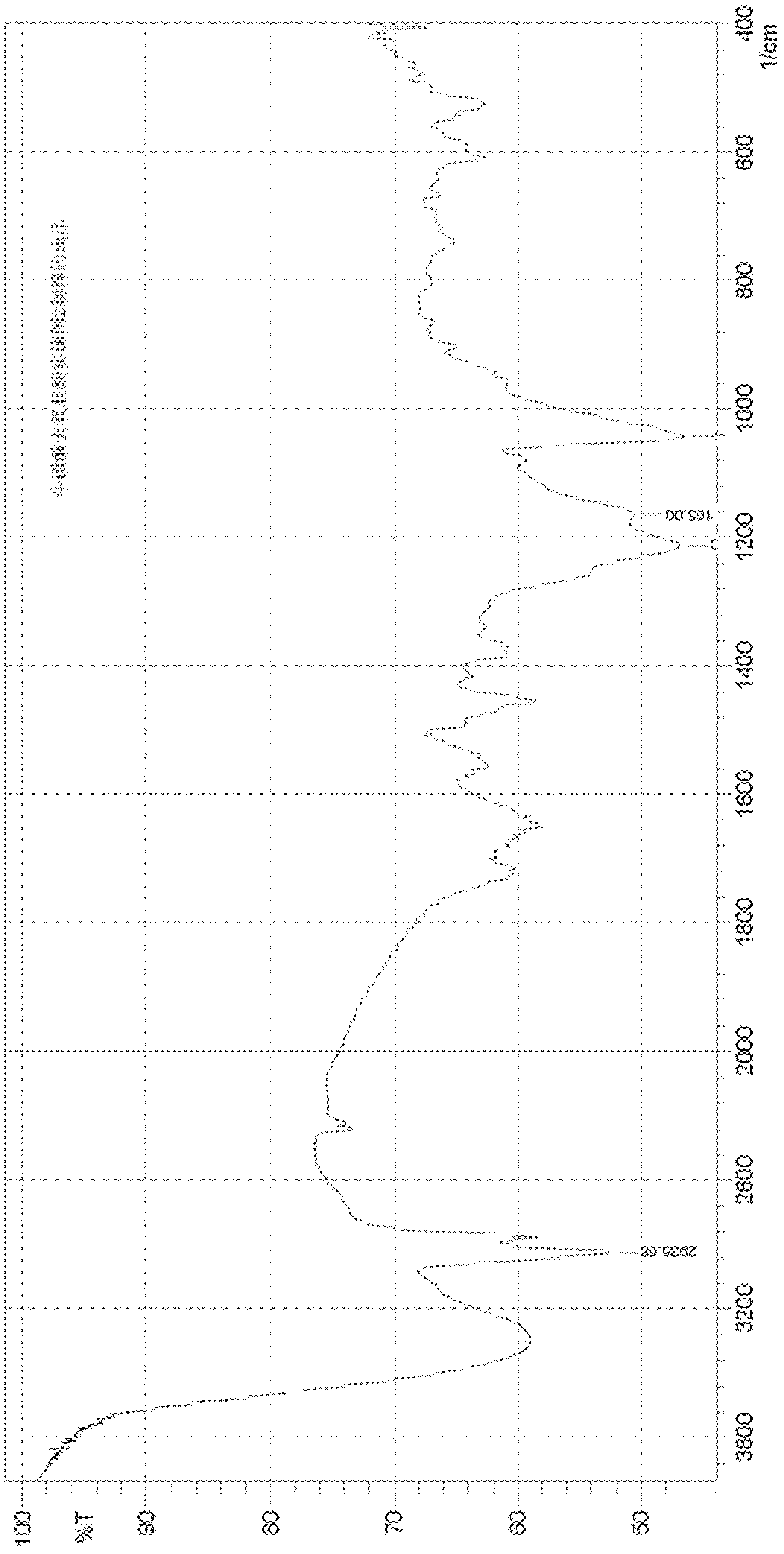
Preparation method of tauro ursodesoxy cholic acid
A technology for tauroursodeoxycholic acid and ursodeoxycholic acid is applied in the field of preparing tauroursodeoxycholic acid, which can solve problems such as environmental pollution and achieve the effects of cheap materials, short reaction time and improved yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 tauroursodeoxycholic acid
[0048] Add 39.2 g (0.1 mol) of ursodeoxycholic acid into 100 ml of N,N-dimethylformamide, and stir to dissolve (stir at 150 rpm for 10 minutes). Cool down to 10°C, add 12.66g (0.11mol) of N-hydroxysuccinimide, stir to dissolve (stir at 135rpm for 20 minutes), add 24.76g (0.12mol) of dicyclohexylcarbodiimide and 4-bis 0.12 g (0.001 mol) of methylaminopyridine, after adding all the materials, stirred and reacted at 12° C. for 2.5 hours at a rotation speed of 135 rpm. The content of ursodeoxycholic acid detected by HPLC was less than 0.1%, and the reaction was stopped. Another 12 g (0.12 mol) of taurine was dissolved in 500 ml of aqueous sodium hydroxide (containing 4.8 g of sodium hydroxide, 0.12 mol) to obtain an aqueous solution of sodium taurine. The above dimethylformamide solution was added to the sodium taurine aqueous solution, and 34ml (0.24mol) of triethylamine was added dropwise. After the dropwise add...
Embodiment 2
[0049]The preparation of embodiment 2 tauroursodeoxycholic acid
[0050] 39.2 g (0.1 mol) of ursodeoxycholic acid was added into 100 ml of tetrahydrofuran, stirred and dissolved (stirred at 150 rpm for 10 minutes). Cool down to 0°C, add 12.66g (0.11mol) of N-hydroxyphthalimide, stir to dissolve (stir at 150rpm for 20 minutes), add 15.14g (0.12mol) of diisopropylcarbodiimide and 0.12 g (0.001 mol) of 4-dimethylaminopyridine, after adding all the materials, react at 8° C. for 20 min, the content of ursodeoxycholic acid detected by HPLC is less than 0.1%, and the reaction is stopped. Another 12 g (0.12 mol) of taurine was dissolved in 500 ml of aqueous sodium hydroxide (containing 4.8 g of sodium hydroxide, 0.12 mol) to obtain an aqueous solution of sodium taurine. The above tetrahydrofuran solution was added to the aqueous sodium taurine solution, and 34ml (0.24mol) of triethylamine was added dropwise. After the dropwise addition, the mixture was reacted at 25°C for 2.5 hours a...
Embodiment 3
[0051] The preparation of embodiment 3 tauroursodeoxycholic acid
[0052] Add 39.2 g (0.1 mol) of ursodeoxycholic acid into 100 ml of dimethyl sulfoxide, and stir to dissolve (stir at 150 rpm for 10 minutes). Cool down to 10°C, add 12.66g (0.11mol) of N-hydroxysuccinimide, stir to dissolve (stir at 150rpm for 10), add 1-ethyl-3-(3-dimethylaminopropyl) carbon di 18.62g (0.12mol) of imine and 012g (0.001mol) of 4-dimethylaminopyridine, after adding all the materials, stir and react at 8°C for 20min, the rotation speed is 150rpm, and the content of ursodeoxycholic acid detected by HPLC is less than 0.1% , stop responding. Another 12 g (0.12 mol) of taurine was dissolved in 500 ml of aqueous sodium hydroxide (containing 4.8 g of sodium hydroxide, 0.12 mol) to obtain an aqueous solution of sodium taurine. The above dichloromethane solution was added to the aqueous sodium taurine solution, 34ml (0.24mol) of triethylamine was added dropwise, and the reaction was carried out at 22°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com