Hybridoma cell lines and antibodies, immunosorbents, immunoaffinity columns and kits and their applications
A hybridoma cell line, immunoadsorbent technology, applied in solid adsorbent liquid separation, antifungal/algae/lichen immunoglobulin, other chemical processes, etc., to achieve the effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] Specifically, the preparation method of hybridoma cell line and monoclonal antibody in the present invention may comprise the following steps:
[0029] (1) Preparation of zearalenone immunogen
[0030] Dissolve 5 mg of zearalenone ZON in 4 mL of pyridine, add 3 mg of O-carboxymethyl hydroxylamine, stir at room temperature for 24 hours, then vacuum-dry, dissolve in water, adjust the pH to 8.0, and extract 3 times with ethyl acetate, Dehydration crystallization. The crystals were dissolved in dioxane to form a 5 mmol / L solution, and 30 mg of BSA was weighed and dissolved in 2 mL of 0.05 mol / L (pH 7.4) PBS. At 4°C, mix the two, and slowly add 3 mg of N-hydroxysuccinimide (NHS) and 6 mg of N, N'-dicyclohexyl in a solution of 0.5 mL of dioxane Carbodiimide (DCC), the resulting mixture was stirred and reacted at room temperature for 24 hours, dialyzed, centrifuged, and frozen for later use.
[0031] (2) Preparation of hybridoma cells and monoclonal antibodies against zeara...
Embodiment 1
[0080] This example is used to prepare zearalenone immunogen and aflatoxin immunogen
[0081] (1) Dissolve 5 mg of zearalenone ZON in 4 mL of pyridine, add 3 mg of O-carboxymethyl hydroxylamine, stir at room temperature for 24 hours, then vacuum-dry, dissolve in water, adjust the pH to 8.0, and extract with ethyl acetate 3 times, dehydration and crystallization. The crystals were dissolved in dioxane to form a 5 mmol / L solution, and 30 mg of BSA was weighed and dissolved in 2 mL of 0.05 mol / L (pH 7.4) PBS. At 4°C, mix the two, and slowly add 3 mg of N-hydroxysuccinimide (NHS) and 6 mg of N, N'-dicyclohexyl in a solution of 0.5 mL of dioxane Carbodiimide (DCC), the resulting mixture was stirred and reacted at room temperature for 24 hours, dialyzed, centrifuged, and frozen for later use. The zearalenone immunogen, ZON-BSA, was obtained.
[0082] (2) 4 mg of aflatoxin B 1 Dissolve in 2 mL of acetone, add 40 μL of 10% HO 2 SO 4 , Stir the reaction at 56°C for 4h; evaporate ...
Embodiment 2
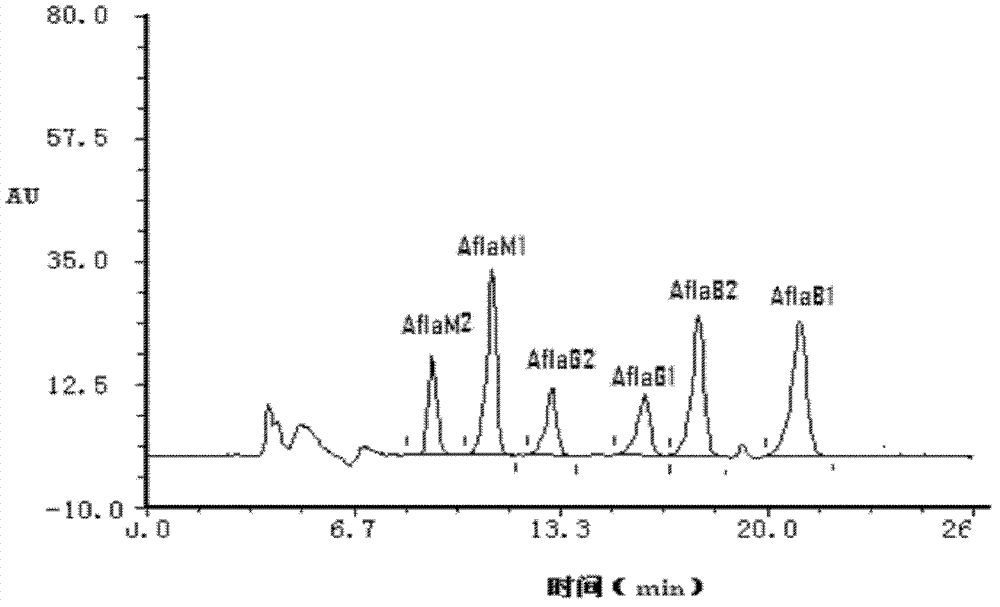
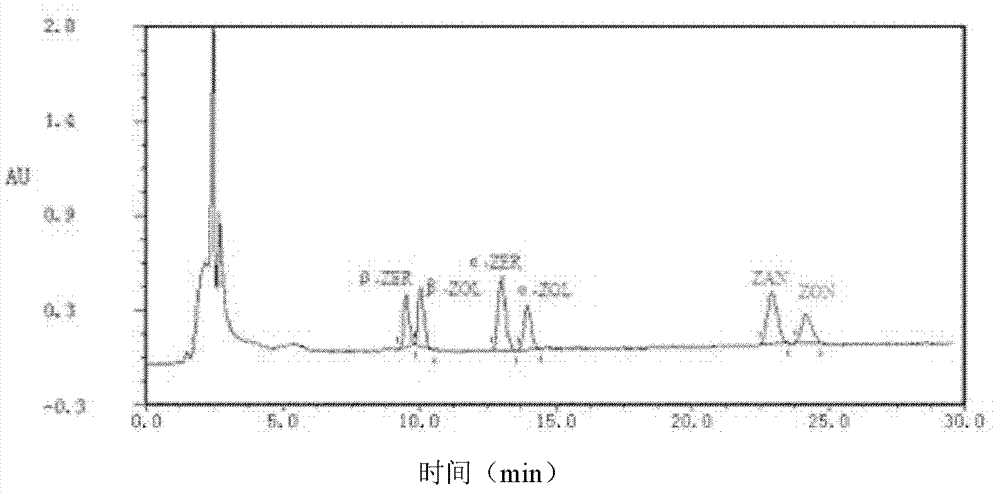
[0085] This embodiment is used for preparing anti-aflatoxin B 1 , B 2 , G 1 , G 2 , M 1 , M 2 and Aspergillus versicolor monoclonal antibodies (antibody A) and monoclonal antibodies against the zearalenone congeners α-ZER, α-ZOL, β-ZER, β-ZOL, ZAN, and ZON (antibody B)
[0086] (1) Animal immunization: the immunized animals are female BALB / c mice about 6-8 weeks old. Five mice were immunized with the zearalenone immunogen. The zearalenone immunogen (100 μg / cause) was added with an equal amount of Freund's complete adjuvant to make an emulsified agent for immunization, and then the adjuvant was changed to an incomplete adjuvant for a total of 6 immunizations with an interval of 2 weeks between each time. Except for the first multi-point subcutaneous injection on the back of the neck, the rest were intraperitoneal injections. After the immunization, the mice were sacrificed, and splenocytes were collected.
[0087] Cell fusion: Splenocytes and hybridoma cells were subjec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com