Aromatase inhibitor high-throughput screening model based on fluorescence detection principle
An aromatase, high-throughput technology, applied in the field of pharmacology, can solve the problems of time-consuming and laborious, high experimental conditions, and difficulty in high-throughput screening.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0021] 1. Detection of aromatase activity
[0022] 1) Experimental materials
[0023] Estradiol detection kit (Cisbio, France), testosterone (Sigma-aldrich, USA), NADPH (Roche, USA), letrozole (Meilon, China), 384 low volume white plate (Corning, USA), human source Aromatase (BD, USA), pipette tip (Axygen, USA).
[0024] 2) Experimental steps
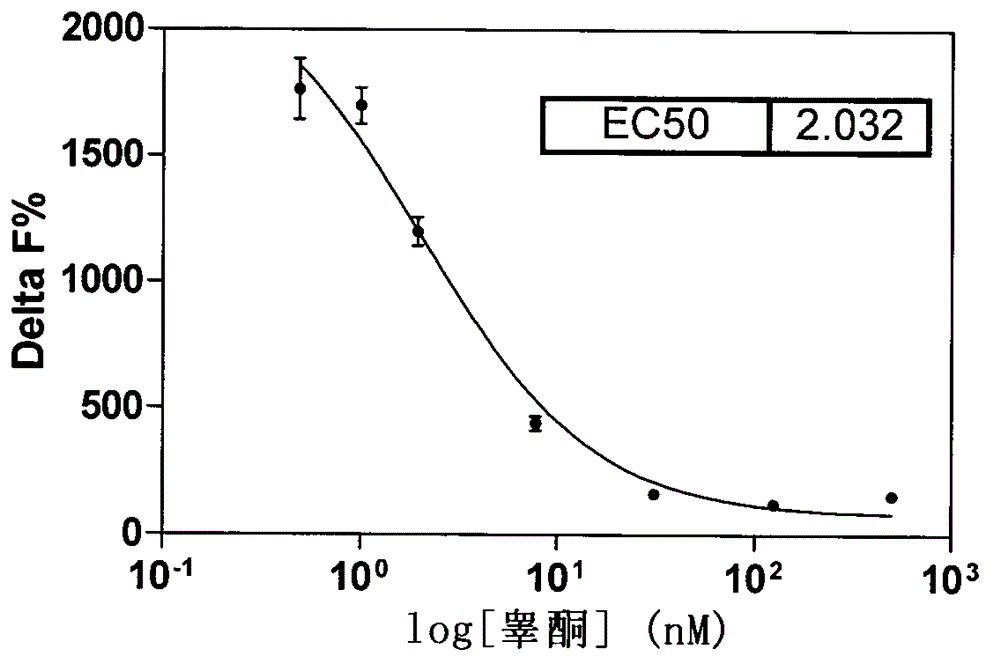
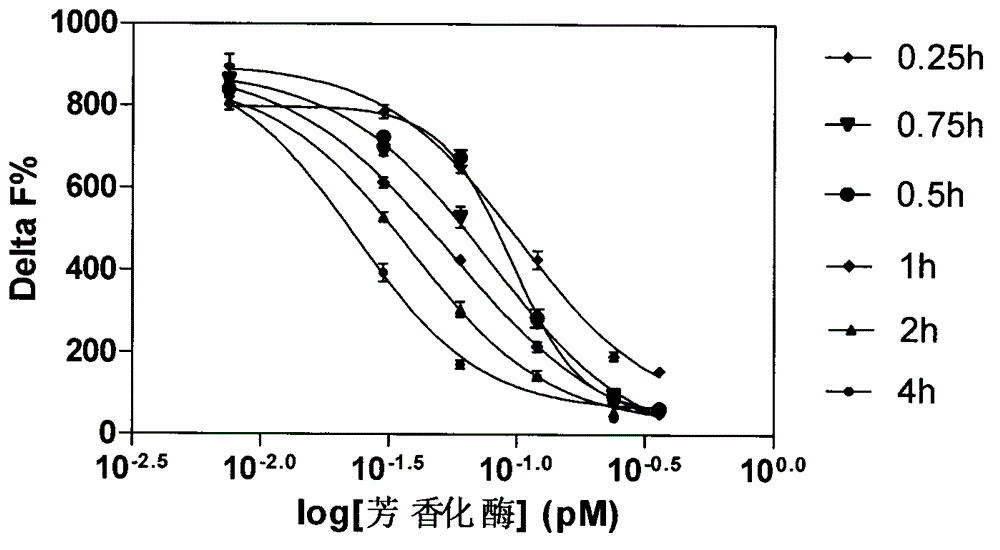
[0025] ●Carry out experiments on aromatase concentration gradient, incubation time, substrate concentration, and NADPH concentration, see Figure 1-4 .
[0026] Precisely weigh the compound to be tested, add DMSO solvent to form a mother solution, and then use the detection buffer to prepare the solution of the compound to be tested to the required concentration, the initial screening concentration is about 1×10 -3 mol / L.
[0027] ● Add 2 μl of aromatase solution, 2 μl of substrate solution, 4 μl of buffer or compound to be screened, and 2 μl of NADPH to each well of the reaction vessel. React at 37°C for 1 hour.
[0028] ●Add Es...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com