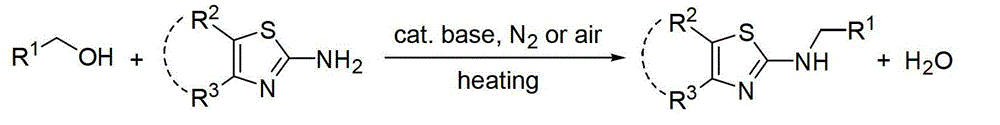Aminothiazole compound dehydration and alkylation method
An aminothiazole and compound technology, applied in the field of synthesis of N-alkylaminothiazole and derivatives thereof, achieves the effects of reducing synthesis cost, good research and industrial application prospects, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of N-(4-methoxybenzyl)-2-aminobenzothiazole by the reaction of 2-aminobenzothiazole and 4-methoxybenzyl alcohol
[0020]
[0021] Add 2-aminobenzothiazole (1mmol), 4-methoxybenzyl alcohol (1.5mmol, 1.5equiv.), NaOH (20mol%) in turn into a 20mL reaction tube, and directly seal and heat to 120°C for 3h under air , The conversion rate of the reaction was 99% as measured by GC-MS. The product was separated and purified by column chromatography, and the separation yield was 95%. 1 HNMR (500MHz, d 6 -DMSO):δ8.44(t,J=5.0Hz,1H),7.67(d,J=8.0Hz,1H),7.41(d,J=8.0Hz,1H),7.32(d,J=9.0Hz ,2H),7.25-7.21(m,1H),7.04-7.01(m,1H),6.92(d,J=8.5Hz,2H),4.53(d,J=5.5Hz,2H),3.74(S, 3H). 13 C NMR (125.4MHz, d 6 -DMSO): δ166.3, 158.6, 152.7, 130.9, 130.6, 129.0, 125.7, 121.07, 121.05, 118.2, 113.9, 55.2, 46.9. MS (EI): m / z (%) 270 (16, M+), 219 ( 4), 207(3), 121(100), 91(5), 77(8), 69(7), 63(5).
Embodiment 2
[0023] Preparation of N-(3-methoxybenzyl)-2-aminobenzothiazole by the reaction of 2-aminobenzothiazole and 3-methoxybenzyl alcohol
[0024]
[0025] Add 2-aminobenzothiazole (1mmol), 3-methoxybenzyl alcohol (1.5mmol, 1.5equiv.), NaOH (20mol%) in sequence to a 20mL reaction tube, and directly seal and heat to 120°C for 3h under air , GC-MS measured the conversion rate of 90%. The product was separated and purified by column chromatography, and the separation yield was 74%. 1 HNMR (500MHz, CDCl 3 ):δ7.55(d,J=8.0Hz,1H),7.38(d,J=8.0Hz,1H),7.26-7.23(m,2H),7.07-7.04(m,1H),6.98-6.93( m,3H),6.82(dd,J=2.5Hz,J=8.5Hz,1H),4.58(s,2H),3.74(s,3H). 13 CNMR (125.4MHz, CDCl 3 ):δ168.0,160.0,152.2,139.1,130.4,129.9,126.0,121.5,120.8,119.9,118.8,113.4,113.2,55.2,49.4.MS(EI):m / z(%)270(76,M+), 269(24), 255(8), 239(5), 136(62), 121(100), 91(49), 78(25), 77(23), 65(19).
Embodiment 3
[0027] Preparation of N-(2-methoxybenzyl)-2-aminobenzothiazole by the reaction of 2-aminobenzothiazole and 2-methoxybenzyl alcohol
[0028]
[0029] 2-Aminobenzothiazole (1mmol), 2-methoxybenzyl alcohol (1.5mmol, 1.5equiv.), NaOH (20mol%) were added sequentially into a 20mL reaction tube, and directly sealed and heated to 120°C for 3h under air , The conversion rate of the reaction was 99% as measured by GC-MS. The product was separated and purified by column chromatography, and the separation yield was 92%. 1 HNMR (500MHz, CDCl 3 ):δ7.54(d,J=8.0Hz,1H),7.46(d,J=8.0Hz,1H),7.36(dd,J=1.0Hz,J=7.5Hz,1H),7.26-7.24(m ,2H),7.06-7.02(m,1H),6.92-6.86(m,2H),6.48(b,1H),4.59(s,2H),3.82(s,3H). 13 CNMR (125.4MHz, CDCl 3):δ167.8,157.5,152.4,130.4,129.4,129.1,125.8,125.6,121.3,120.7,120.5,118.7,110.3,55.3,45.4.MS(EI):m / z(%)274(52,M+), 270(37),255(11),239(42),207(14),151(10),131(22),121(88),119(13),105(8),91(100), 77(13),69(32),65(22),
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com