Photosensitive medicinal preparation containing amino poly-carboxylic acid modification tetraphenylporphyrin compound and purpose thereof
A technology of tetraphenylporphyrin and amino polycarboxylic acid, which is applied in the field of medicine, can solve problems such as poor solubility, influence on the stability of curative effect, and molecular polarity reduction, and achieve broad application prospects, high fluorescence quantum yield and Effects of optical stability, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
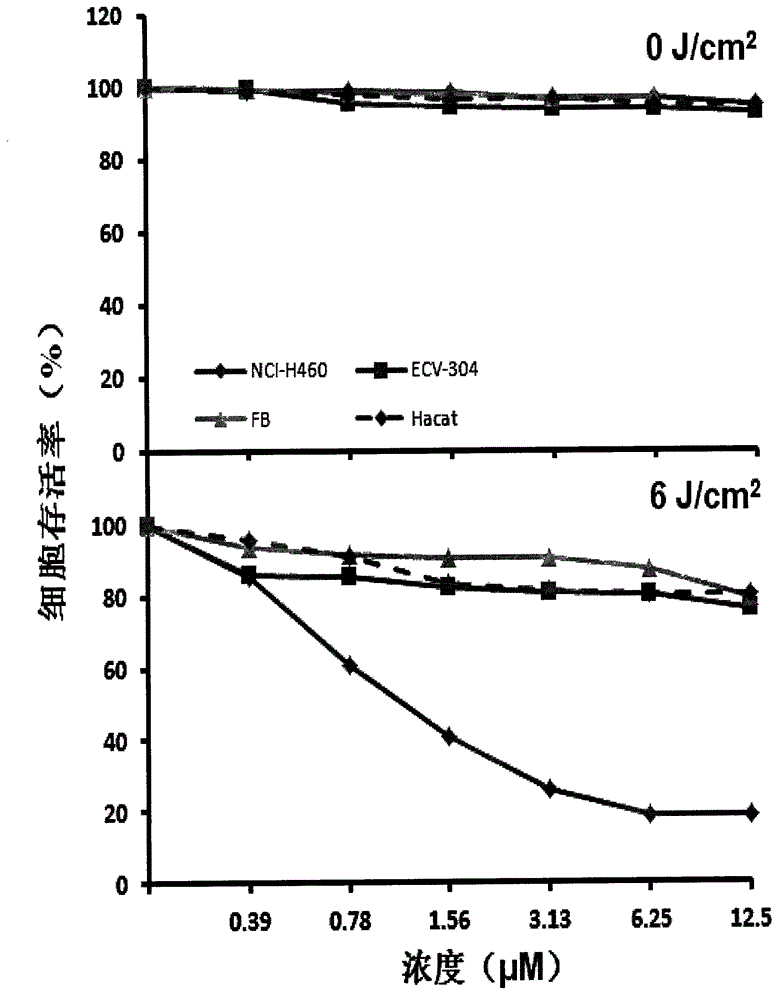
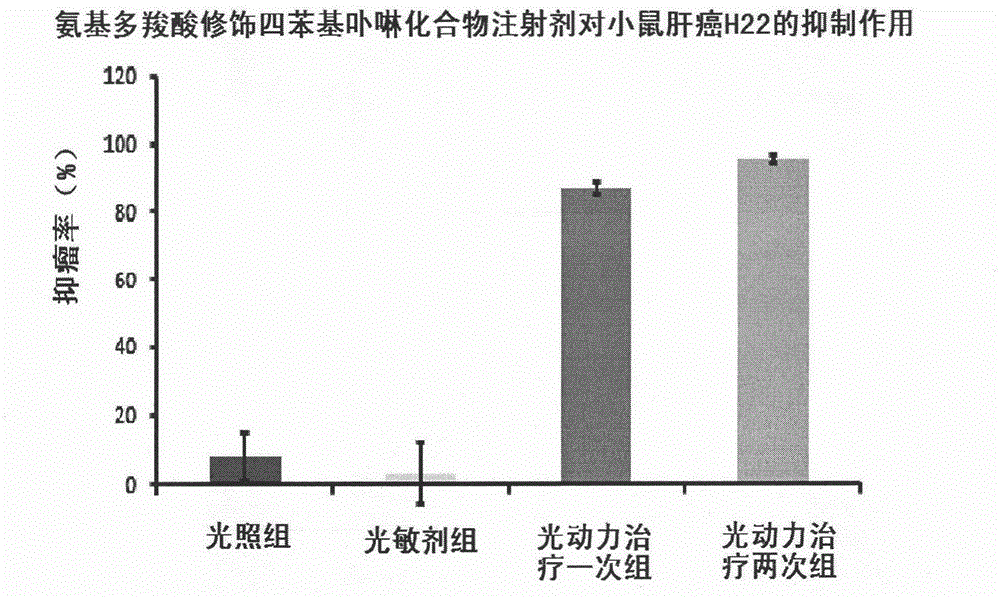
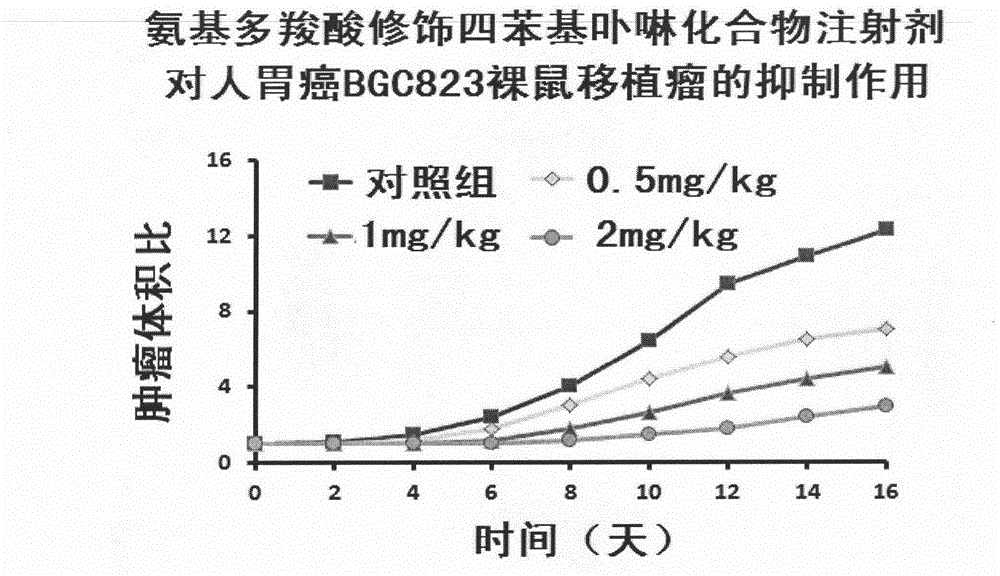
Image
Examples
Embodiment 1
[0026] Example 1 tablet
[0027] Get 0.3kg amino polycarboxylic acid modified tetraphenylporphyrin compound, add 0.5kg starch, 0.1kg magnesium stearate, 0.2kg carboxymethyl cellulose, 2.7kg ethanol (volume percentage composition is 70%), 2.5kg Microcrystalline cellulose and 3.2kg cornstarch are mixed thoroughly to form wet granules, dried at 60-70°C for 2-4 hours, and pressed into tablets. Tablets are made into tablets. The excipients used as excipients include magnesium sulfate, corn starch, and talcum powder. The specifications are: 5mg, 10mg, 20mg, 50mg, and 100mg.
[0028] In practice, the tetraphenylporphyrin compound modified with aminopolycarboxylic acid is the compound represented by the following formula (I) and its pharmaceutically acceptable salts such as potassium salt, sodium salt, ammonium salt and the like.
[0029]
[0030] Among them, R 1 = H, OH, OCH 3 ;
[0031]
Embodiment 2
[0032] Embodiment 2 capsules
[0033] Get 0.3kg amino polycarboxylic acid modified tetraphenylporphyrin compound, add 0.02kg magnesium stearate, 0.2kg carboxymethyl cellulose, 0.48kg microcrystalline cellulose, fully stir and mix to make wet granules, and make the The wet granules are directly dried at 60-70° C. for 2-4 hours, and then filled into empty capsule shells, and each capsule contains 20 mg of amino polycarboxylic acid modified tetraphenylporphyrin compound.
Embodiment 3
[0034] Embodiment 3 sustained and controlled release tablet
[0035] Get 0.3kg aminopolycarboxylic acid modified tetraphenylporphyrin compound, add 0.07kg hydroxypropyl methylcellulose, 0.25kg carboxymethylcellulose, 0.37kg ethanol (volume percentage is 70%), 0.1kg hard Magnesium fatty acid, etc. are fully stirred and mixed to form wet granules, dried at 60-70°C for 2-4 hours, and pressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com