Method for preparing lafutidine from hydroxylamine hydrochloride
A technology of hydroxylamine hydrochloride and lafutidine, which is applied in the direction of organic chemistry, can solve problems such as increasing costs, and achieve the effects of improving safety, improving product quality and total yield, and increasing synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
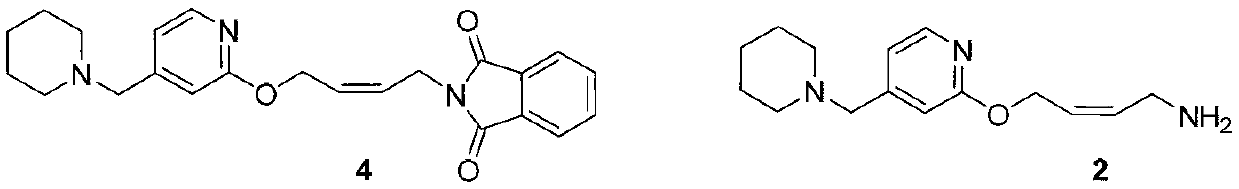
[0039] Embodiment 1: N-[cis-4-[4-(N-piperidinylmethyl)pyridine-2-oxygen]-2-buten-1-yl]phthalimide (compound of formula 4) preparation of
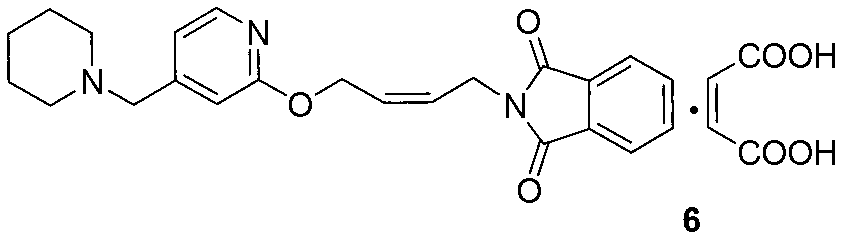
[0040] Take 100g (0.197mol) of maleate salt (compound of formula 5), add 1 liter of water, stir at room temperature for 15 minutes, add sodium hydroxide to adjust the pH to about 12, extract with 500mL of dichloromethane, separate layers, and then use 250mL of dichloromethane Extract with methyl chloride, combine the organic phases, dry over anhydrous sodium sulfate, filter, and evaporate the solvent to give 74.7g of viscous liquid (Compound 4), with a yield of 96.8% (based on crude product)
Embodiment 2
[0041] Example 2: Preparation of N-[cis-4-[4-(N-piperidinylmethyl)pyridine-2-oxygen]-2-butene-1-amine (compound of formula 2)
[0042]In a 500mL three-necked flask, 20 g (0.05 mol) of the imine (the compound of formula 4 prepared in Example 1), 94 g of ethanol, and 21.4 g (0.31 mol) of hydroxylamine hydrochloride were added to produce a large amount of white solid, and then aqueous sodium hydroxide ( Contains sodium hydroxide 51g, 1.27mol), react at room temperature for about 1 hour. After the TLC detection reaction was complete, add 400 mL of water to the reaction solution, extract 200 mL × 2 with dichloromethane, combine the organic phase, wash 200 mL with water, dry the organic phase with anhydrous sodium sulfate, filter, and concentrate to dryness to obtain 13.2 g of light yellow oil ( Compound 2) has a yield of 95.5%, HPLC 98.9%, and is used in the next step without further purification.
Embodiment 3
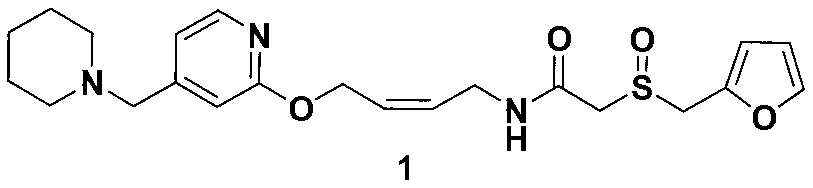
[0043] Example 3: (+ / –)-2-[(2-furylmethyl)sulfinyl]-N-[4-[4-(1-piperidinylmethyl)-2-pyridyl]oxy Synthesis of Sub-(Z)-2-butenyl]acetamide (compound of formula 1, lafutidine)
[0044] Add 70 mL of ethyl acetate to 13.2 g (0.05 mol) of the oily enamine (compound of formula 2) obtained in Example 2, stir to dissolve, and then add p-nitrophenol 2-furan methanesulfonyl acetate (compound of formula 3 ) 15.6g (0.05mol, HPLC purity 99.5%), stirred and reacted at room temperature for 3-4 hours, TLC detected that the reaction was complete. Add 100 mL of water, adjust the pH to 4 with concentrated hydrochloric acid, filter, and separate layers. Add 150 mL of dichloromethane to the water phase, add potassium carbonate to adjust the pH to about 10, and separate layers. Add 80 mL of dichloromethane to the water layer for extraction. Combine the organic phases, wash with 30% potassium carbonate solution (100mL×4), wash with water (100mL×5), separate the organic phase, dry over anhydrous sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com