Medicine composition used for treating hepatitis
A composition and drug technology, applied in the direction of drug combination, medical formula, plant raw materials, etc., can solve the problems of long treatment cycle, high recurrence rate, and single effect, and achieve non-toxic and side effects, good therapeutic effect, and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
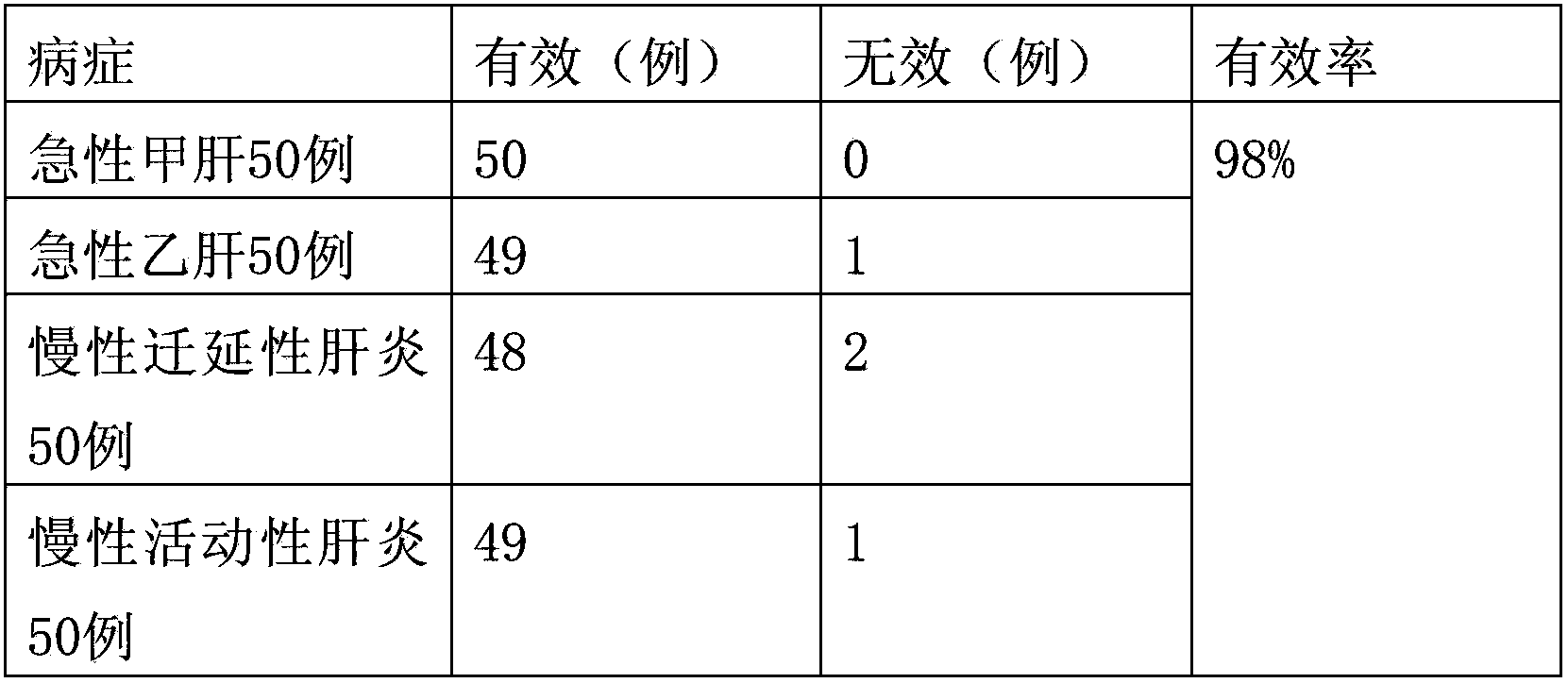
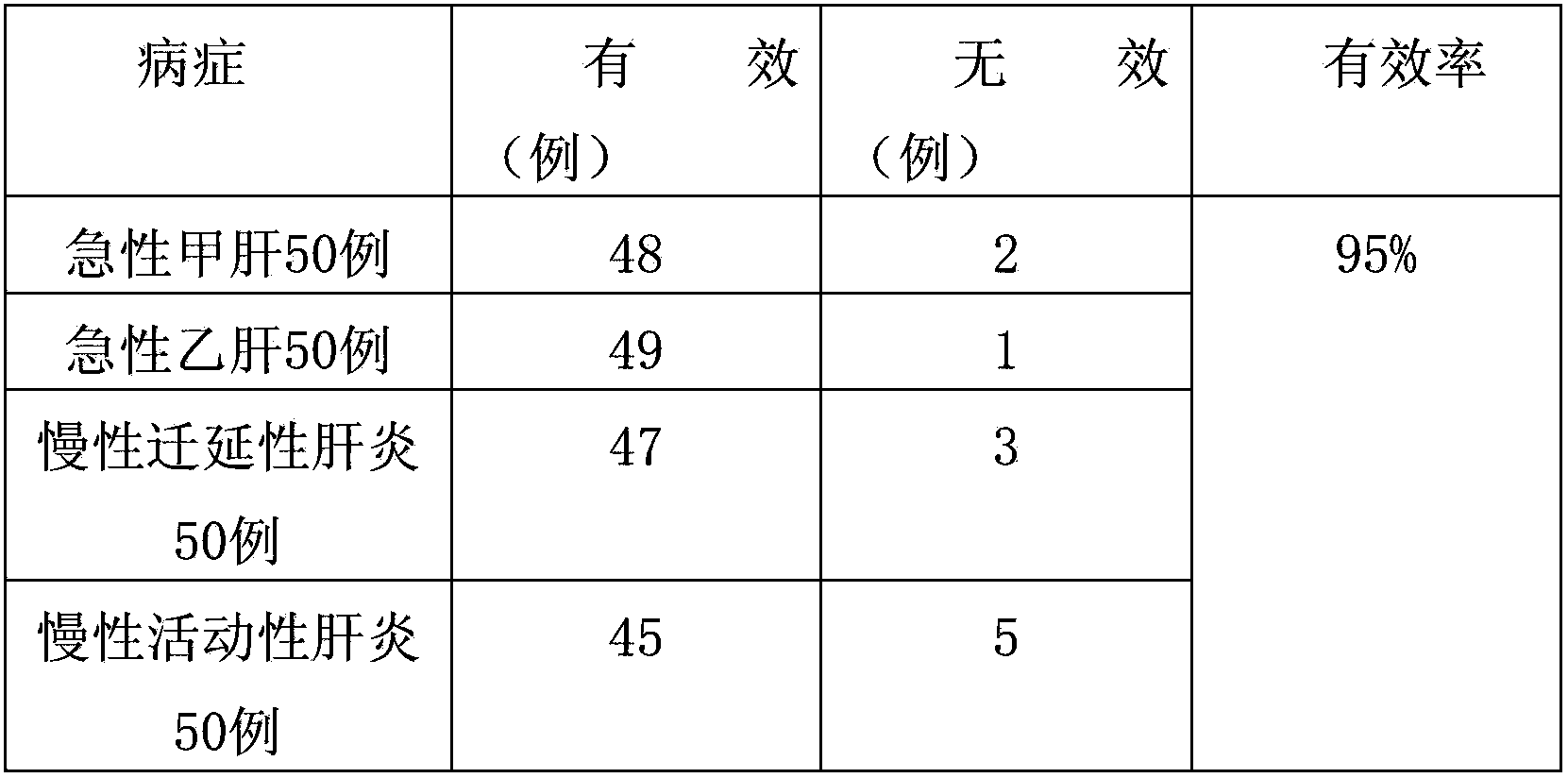
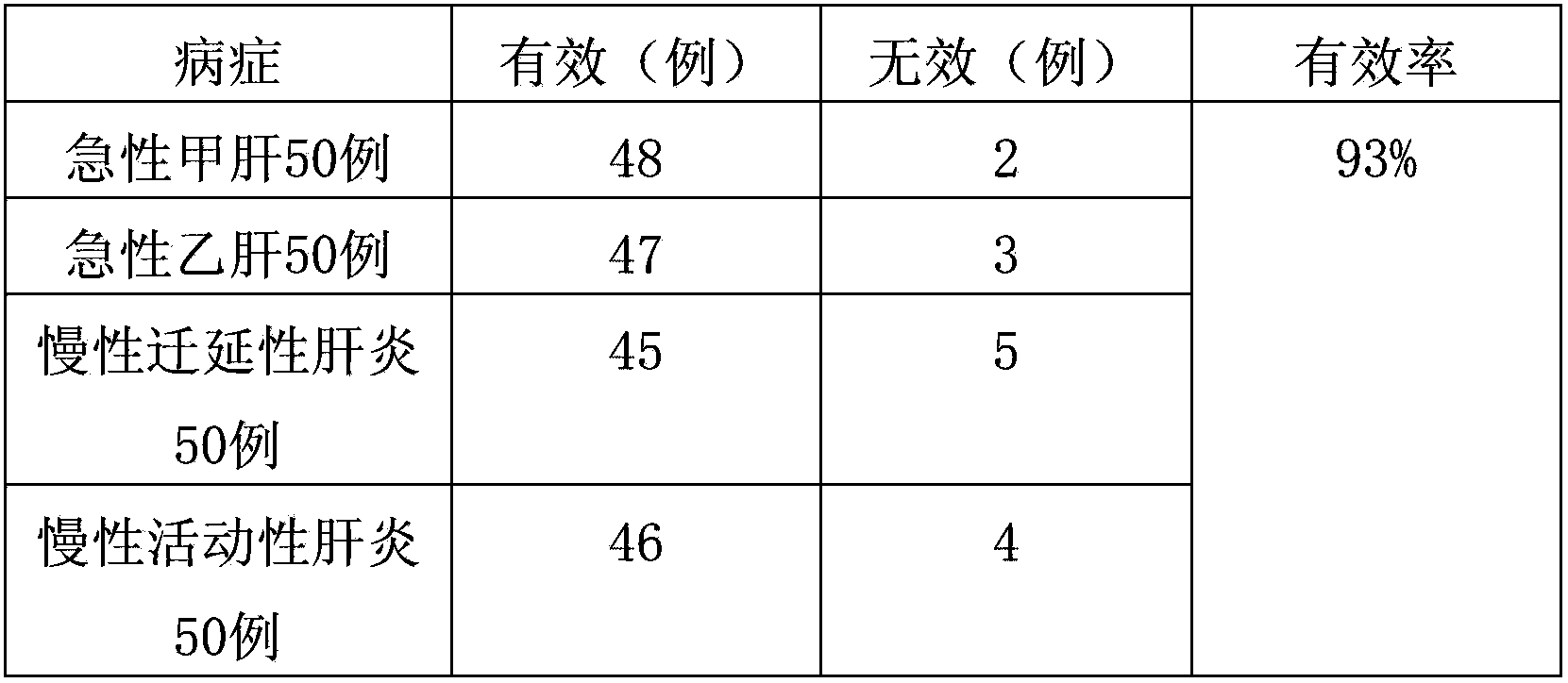
experiment example 1
[0029] Observation object and method
[0030] 1. Case selection
[0031] (1) Etiological diagnosis
[0032] Type A: Those who have any one of the following four items:
[0033] 1. Serum anti-HAVIgM positive in patients with acute hepatitis;
[0034] 2. The anti-HAVIgG titer of the double serum in the acute phase and the recovery phase increased by more than 4 times;
[0035] 3. HAV particles were detected by anti-HAV immuno-electron microscopy in feces in the early acute stage;
[0036] 4. HAAg was found in feces in the early acute stage.
[0037] Type B: Those who have any one of the following four items:
[0038] 1. Serum HbsAg positive, or HbeAg positive;
[0039] 2. Serum HbsAg positive, but anti-HbcIgM positive, or anti-HBs, or anti-HBc positive;
[0040] 3. Serum HBV-DNA or DNA polymerase positive, or HbeAg, or anti-Hbe positive;
[0041] 4. The indicators of HBV infection are not obvious or only one indicator of anti-HBc is positive, while HbcAg, HbsAg or HBV-DN...
experiment example 2
[0117] Experimental Example 2: Pharmacodynamics Experiment
Embodiment 1
[0131] Embodiment 1 capsule
[0132] Kelp extract 3kg Eucheuma extract 1kg
[0133] Morinda officinalis 50kg, 30kg of scorpion vine.
[0134] Add 15 times the amount of water to Morinda officinalis and Tachibana, soak for 36 hours; decoct twice with slow fire, the first time for 2 hours, filter the liquid medicine for later use, add 10 times the amount of water for the second time, and use Simmer for 1 hour, filter the medicinal liquid for use; combine the secondary medicinal liquids for filtration, and collect the filtrate; concentrate the filtrate to a thick paste with a relative density of 1:1.25-1.35, and bake the thick paste at low temperature for 10 hours to obtain Dry cream powder, mix the dry cream powder with kelp extract and Eucheuma extract: add excipients at a weight ratio of 1:0.5 to starch, granulate, dry at 50°C, granulate and pack into capsules. Capsule specification: 0.2g / grain, 2 tablets of Example 1 a day
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com