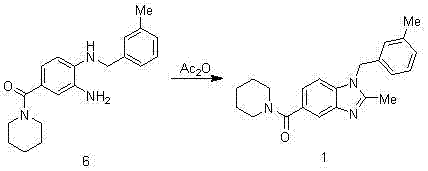
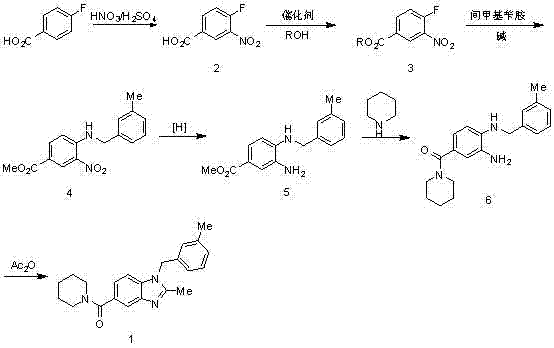
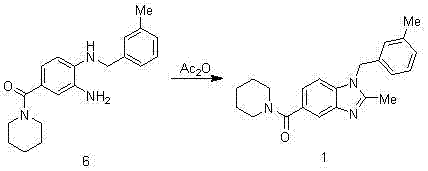
Synthesis method of 2,3-benzopyrrole compound NPS-1577
A technology of NPS-1577 and compounds, applied in the direction of organic chemistry, can solve the problems of high cost of preparation of reaction substrates, difficult control of reaction conditions, expensive raw materials and other problems, and achieve easy industrial scale-up production, cheap raw materials, and easy access to raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]
[0058] Add 300ml of concentrated sulfuric acid to the reaction bottle, add 100g of p-fluorobenzoic acid in batches, cool in an ice bath to below 10°C, add 71.5ml of concentrated nitric acid dropwise to control the temperature at 0-5°C, and raise the temperature to 50-55°C for 2.5 hours after dropping. TLC monitors that the reaction of the raw materials is completed and cooled down. The reaction solution is poured into 810ml of ice water, stirred for 0.5h, suction-filtered, and air-dried at 40°C to obtain 120g of intermediate 2 as a pale yellow solid. 1 H-NMR (400 MHz, CDCl 3 ) δ: 11.10(s, 1H), 8.83 (dd, J = 2 Hz, 1H), 8.42 (m, 1H), 7.46 (t, 1H).
[0059]
[0060]
[0061] Add 2000ml of methanol and 500g of intermediate 2 to the reaction bottle in sequence, cool in an ice bath to 0°C, add 800ml of thionyl chloride dropwise (the dropwise addition is completed within 1 hour), heat up to 65°C and reflux for 1 hour after dropping, TLC shows that the reaction of th...
Embodiment 2
[0072]
[0073] Add 300ml of concentrated sulfuric acid to the reaction bottle, add 100g of p-fluorobenzoic acid in batches, cool in an ice bath to below 10°C, add 71.5ml of concentrated nitric acid dropwise to control the temperature at 5-10°C, and raise the temperature to 25-30°C for 6 hours after dropping, TLC After monitoring the completion of the raw material reaction and cooling, the reaction solution was poured into 810ml of ice water, stirred for 0.5h, suction filtered, and air-dried at 40°C to obtain 118g of intermediate 2 as a pale yellow solid. 1 H-NMR (400 MHz, CDCl 3 ) δ: 11.10(s, 1H), 8.83 (dd, J = 2 Hz, 1H), 8.42 (m, 1H), 7.46 (t, 1H).
[0074]
[0075]
[0076] Put 1600ml of methanol and 400g of intermediate 2 into the reaction bottle in sequence, cool in an ice bath to 0°C, add 80g of concentrated sulfuric acid dropwise (completely added within 1 hour), and heat up to 65°C for 1 hour under reflux after dropping. The solvent was evaporated to obtain 3...
Embodiment 3
[0087]
[0088] Add 600ml of concentrated sulfuric acid to the reaction flask, add 200g of p-fluorobenzoic acid in batches, cool in an ice bath to 0-5°C, add 143ml of concentrated nitric acid dropwise to control the temperature at 5-10°C, and raise the temperature to 55-65°C for 1 hour after dropping, TLC After monitoring the completion of the raw material reaction and cooling, the reaction liquid was poured into 1620ml of ice water, stirred for 1 hour, suction-filtered, and air-dried at 40°C to obtain 240g of intermediate 2 as a pale yellow solid. 1 H-NMR (400 MHz, CDCl 3 ) δ: 11.10(s, 1H), 8.83 (dd, J = 2 Hz, 1H), 8.42 (m, 1H), 7.46 (t, 1H).
[0089]
[0090]
[0091]Put 1600ml of ethanol and 400g of intermediate 2 into the reaction bottle in sequence, cool in an ice bath to 0°C, add 80g of concentrated sulfuric acid dropwise (completely added within 1 hour), and raise the temperature to 78°C for 1 hour under reflux after dropping, TLC shows that the reaction of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com