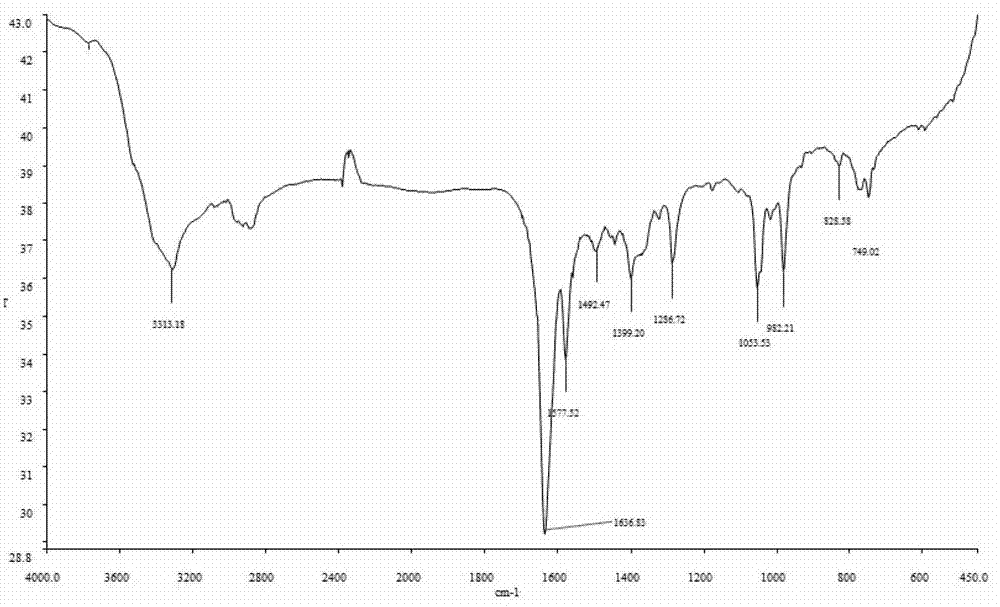
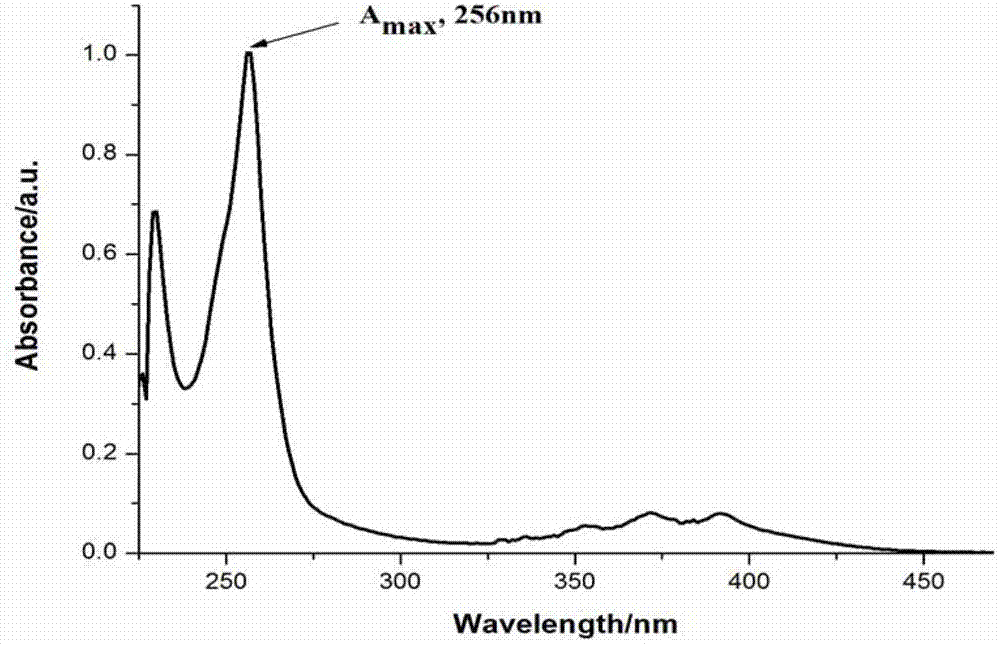
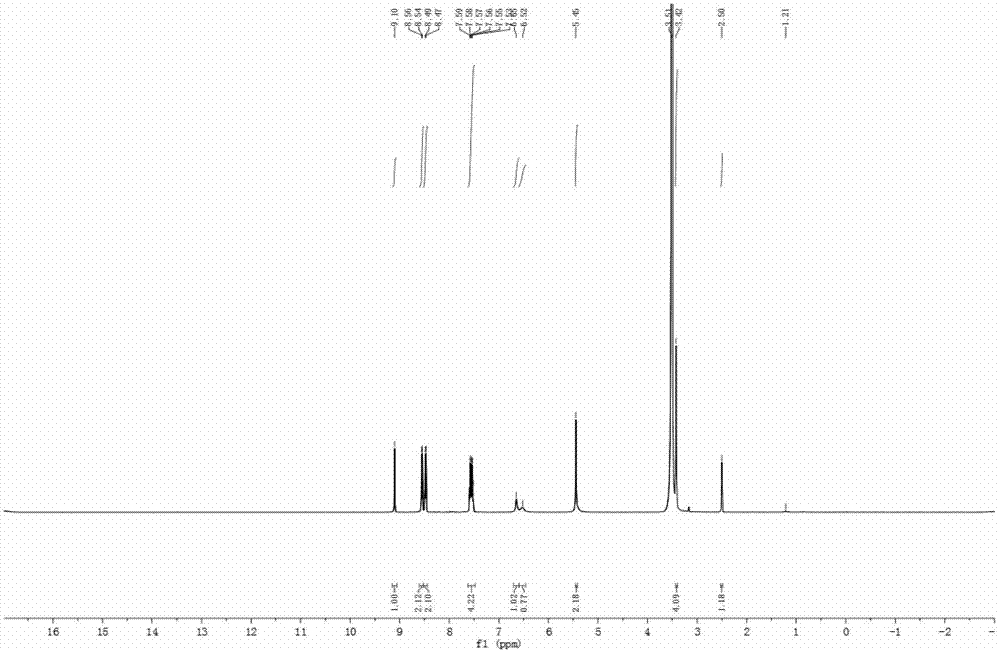
9-hydroxymethyl-10-imidazolanthracenehydrazone, and synthetic method and application thereof
A synthetic method, hydroxymethyl technology, applied in the field of medicine, can solve problems such as no synthetic method of 9-hydroxymethyl-10-imanthrene, achieve good potential medicinal value, reduce liver and kidney toxicity, and anti-tumor active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1) Dissolve 0.01mol of 9,10-anthracenedicarbaldehyde and 0.01mol of 4,5-dicyanimidazole-2-hydrazine hydrogen bromide in 50mL of absolute ethanol, and reflux at 78°C until complete (TLC Tracking detection, about 3h), stop the reaction, cool the reaction solution to room temperature, let it stand for 1h, precipitate an orange solid, filter, and dry the obtained solid in vacuum at room temperature for 10h to obtain a mixed product with a yield of 55%;
[0034] 2) The product A was subjected to silica gel column chromatography, eluted with a mixed solvent composed of anhydrous methanol and dichloromethane with a volume ratio of 1:50, tracked and detected by thin layer chromatography, and the eluate containing the target component was collected. The obtained eluate is concentrated and dried to obtain the target intermediate;
[0035] 3) Take 0.01mol of the target intermediate, heat it with 30mL of anhydrous methanol and dissolve it into a clear solution; take 0.05mol of NaBH...
Embodiment 2
[0045] 1) Dissolve 0.01mol of 9,10-anthracenedicarbaldehyde and 0.01mol of 4,5-dicyanimidazole-2-hydrazine hydrogen bromide in 100mL of anhydrous methanol, and reflux at 70°C until complete (TLC Tracking detection, about 3h), stop the reaction, cool the reaction solution to 20°C, let it stand for 2h, precipitate an orange solid, filter, and dry the obtained solid in vacuum to obtain product A with a yield of 65%;
[0046] 2) The product A was subjected to silica gel column chromatography, eluted with a mixed solvent composed of anhydrous methanol and chloroform with a volume ratio of 1:70, tracked and detected by thin layer chromatography, and the eluate containing the target component was collected, and the obtained eluted Deliquoring, concentrating and drying to obtain the target intermediate;
[0047] 3) Take 0.01mol of the target intermediate, heat it with 20mL of anhydrous methanol and dissolve it into a clear solution; take 0.02mol of NaBH 4 , dissolved into a clear sol...
Embodiment 3
[0049] 1) Dissolve 0.01mol of 9,10-anthracenedicarbaldehyde and 0.01mol of 4,5-dicyanimidazole-2-hydrazine hydrogen bromide in 80mL of absolute ethanol, and reflux at 120°C until complete (TLC Tracking detection, about 3h), stop the reaction, cool the reaction solution to 25°C, let it stand for 4h, precipitate an orange solid, filter, and dry the obtained solid in vacuum to obtain product A with a yield of 60%;
[0050] 2) The product A was subjected to silica gel column chromatography, eluted with a mixed solvent composed of anhydrous methanol and ethyl acetate with a volume ratio of 1:100, tracked and detected by thin layer chromatography, and the eluate containing the target component was collected. The obtained eluate is concentrated and dried to obtain the target intermediate;
[0051] 3) Take 0.01mol of the target intermediate, heat it with 30mL of anhydrous methanol and dissolve it into a clear solution; take 0.015mol of NaBH 4 , dissolved in 5mL of anhydrous methanol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com