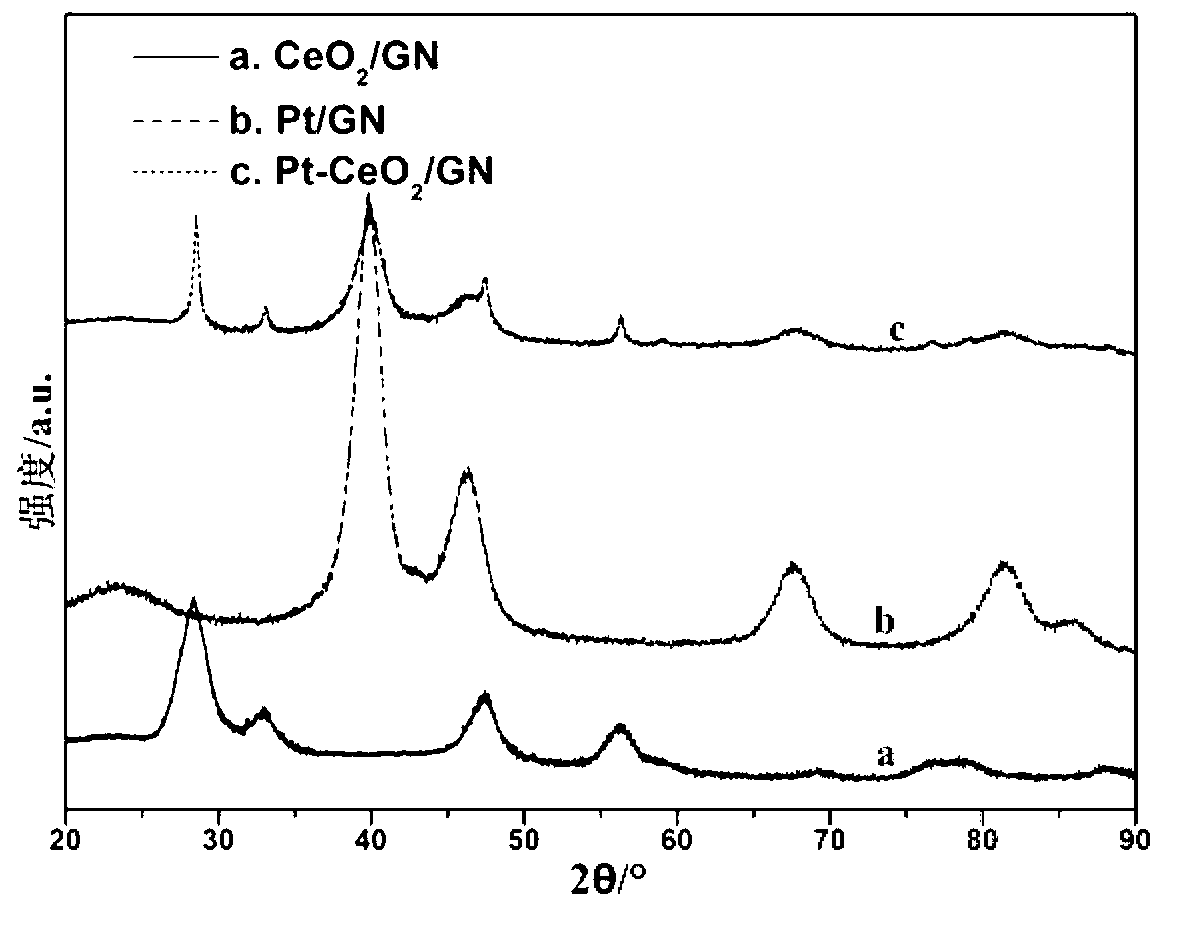
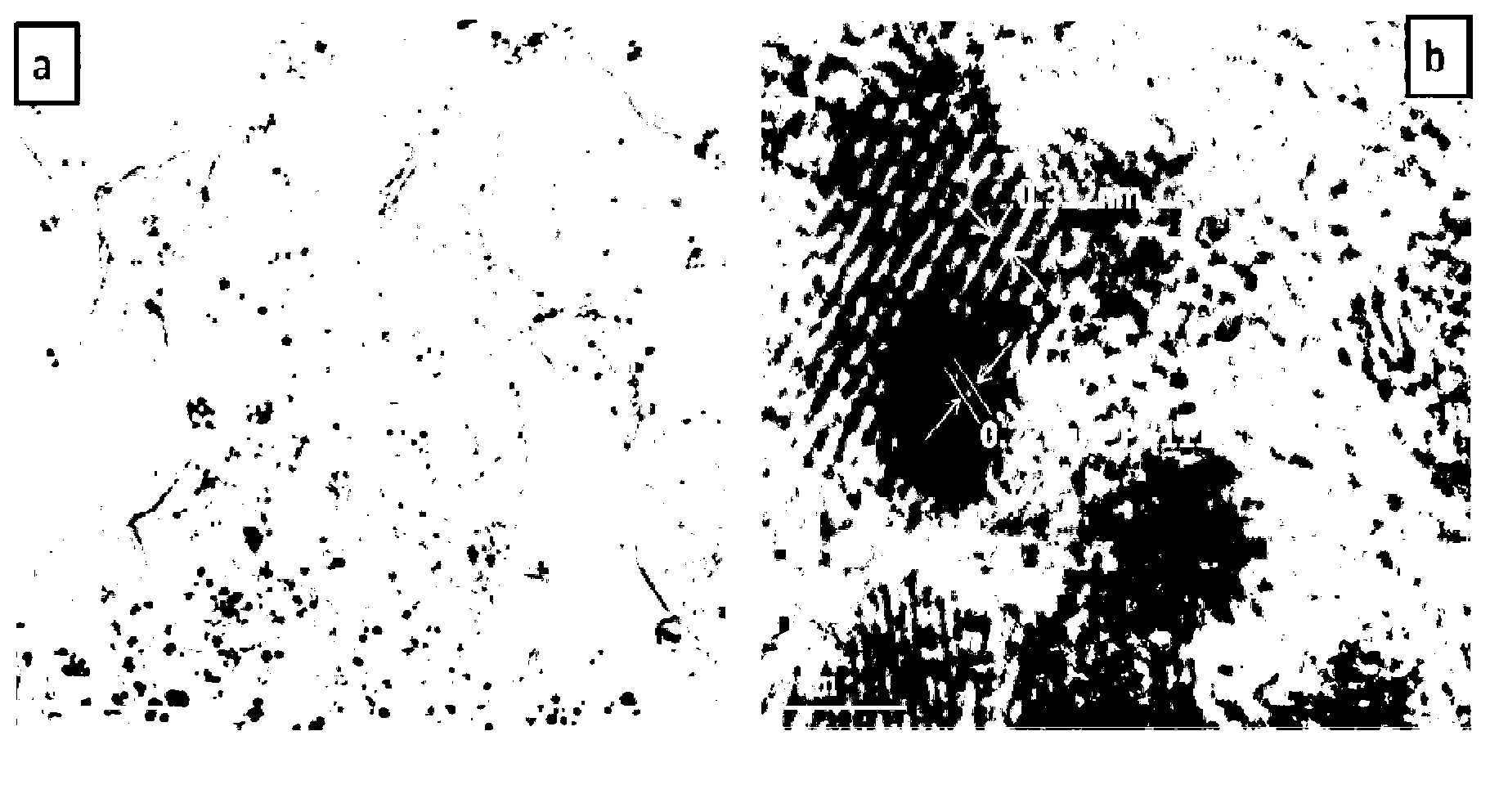
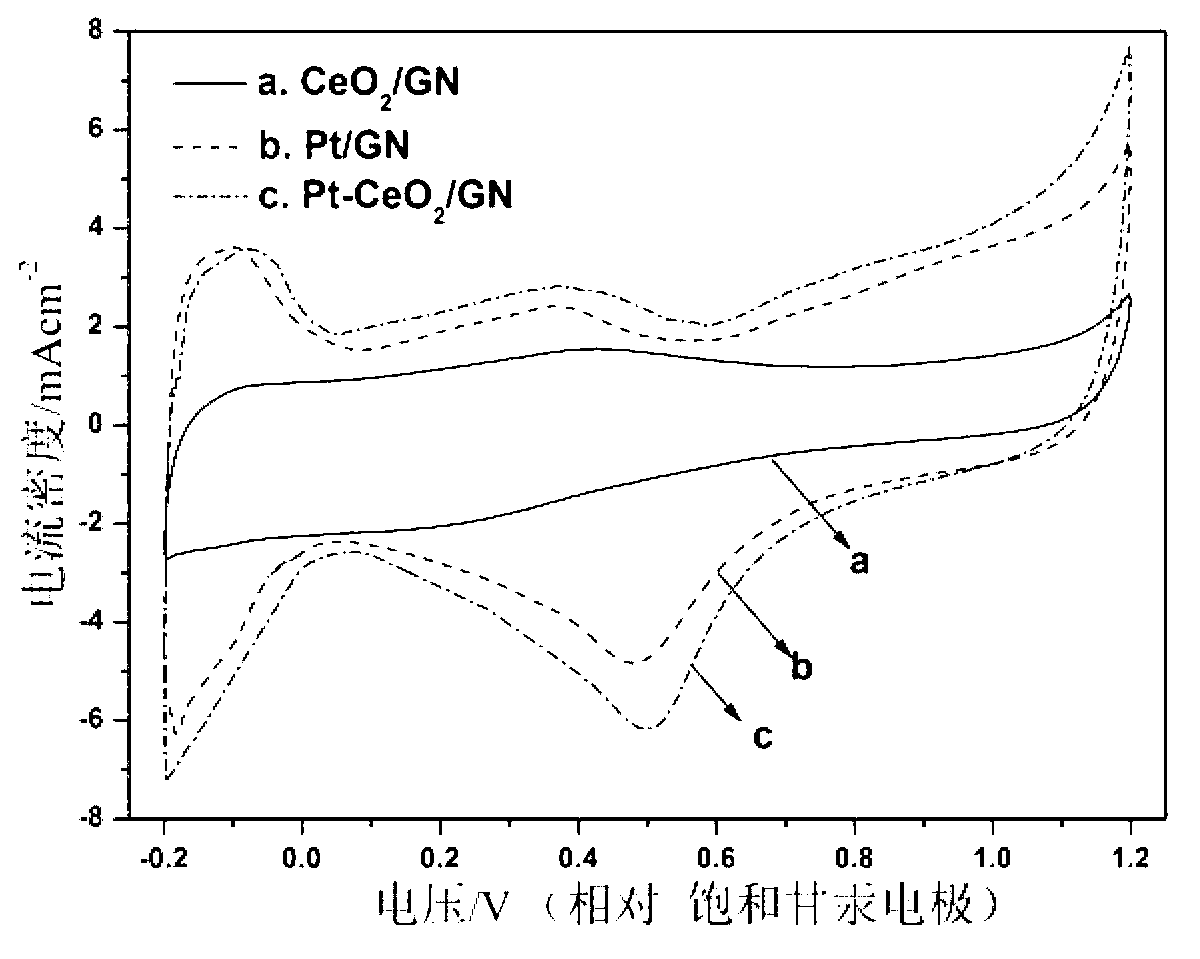
Fuel cell catalyst and preparation method thereof
A fuel cell and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of slow reaction kinetics, high price, low reaction rate, etc., to promote redox reactions and reduce prices Low cost and high oxygen storage capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A. Weigh 2g graphite powder, add 46ml concentrated H 2 SO 4 , 0.5g NaNO 3 and 6g KMnO 4 , Control the reaction temperature below 10°C and stir the reaction for 1h. The three-neck flask was moved to a 35°C water bath for 0.5h reaction, and 92ml of deionized water was slowly added dropwise. Rapidly raise the temperature to 95°C for 0.5h, add 20ml of 30% H 2 o 2 Stop responding. The product was neutralized by washing with 5% hydrochloric acid solution and deionized water, and dried at 80°C to obtain graphite oxide.
[0025] B. Take 90mg of graphite oxide and 25.2mg of cerium nitrate and ultrasonically disperse them in 100ml of ultrapure water, ultrasonicate for 30min, move the three-necked bottle into an oil bath, raise the temperature to 80°C, and use 0.1mol L -1 Adjust the pH of the solution with ammonia water to 8, reflux at a constant temperature for 4 hours, pour the solution into the reaction kettle while it is hot, and react at 120°C for 4 hours. The product...
Embodiment 2
[0029] A. step is the same as embodiment 1
[0030] B. Take 95mg of graphite oxide and 12.6mg of cerium nitrate and ultrasonically disperse them in 100ml of ultrapure water, ultrasonically for 1h, move the three-necked bottle into an oil bath, raise the temperature to 120°C, adjust the pH of the solution to 11 with ammonia water, and reflux at constant temperature for 6h , Pour the solution into the reaction kettle while it is hot, and react at 160°C for 6h. The product was suction filtered, washed, and dried under vacuum at 50°C to obtain CeO 2 / GN.
[0031] C. Weigh 44.8mg CeO 2 / GN into the three-necked bottle, add 40ml of ethylene glycol and 30mg of chloroplatinic acid, ultrasonically disperse for 2 hours, move the three-necked bottle into an oil bath at 120°C, and adjust the pH to 11 with ethylene glycol solution with a concentration of 5% sodium hydroxide, Stir and reflux for 6 hours, filter and wash to obtain Pt-CeO 2 / GN Catalyst
Embodiment 3
[0033] A, B step are the same as embodiment 1
[0034] C. Weigh 44.8mg CeO 2 / GN into a three-neck bottle, add 50ml ethylene glycol and 30mg chloroplatinic acid, ultrasonically disperse for 2 hours, move the three-necked bottle into a 90°C oil bath, adjust the pH to 10 with 5% potassium hydroxide ethylene glycol solution, Stirred and refluxed for 6h, filtered and washed to obtain Pt-CeO 2 / GN Catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Active area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com