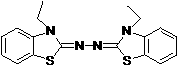
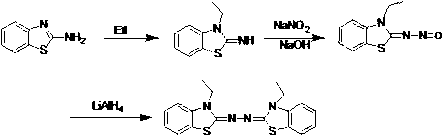
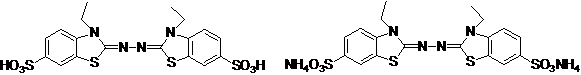Synthetic method for 2, 2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
A technology of benzothiazole and synthesis method, which is applied in the field of synthesis of 2,2'-azino-didiammonium salt, can solve the problems that it is not suitable for industrial production of ABTS, does not conform to environmental friendliness and green chemical atom economy, etc. Achieve the effect of simple operation, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] step one:
[0040] Mix 48.47g of N-ethylaniline with 60ml of H 2 O was added to a 500ml three-necked flask, stirred at room temperature for 5 minutes, then 40ml of concentrated hydrochloric acid was added to the mixture, and 116.62g of KSCN was added, stirred for 10min, then heated to 95°C for reflux reaction for 18h. As the temperature increased, the system The color turns from red to orange and finally to yellow. Cool the reflux system in an ice bath, filter with suction, and 2 O wash, followed by one wash with ethanol. The obtained yellow crude product was recrystallized from ethanol, and then suction filtered to obtain 61.66 g of white powdery solid, with a yield of 94%.
[0041] Step two:
[0042] Put 100 grams of N-ethyl-N-phenylthiourea in a 500ml three-necked flask with 75mlCHCl 3 Dissolve, then add 29ml of elemental bromine in CHCl dropwise with a constant pressure dropping funnel 3 (30ml) mixed solution, control the temperature at 0-20°C, after the dropw...
Embodiment 2
[0050] step one:
[0051] Mix 48.47 g (0.4 mol) of N-ethylaniline with 60 ml H 2 O was added to a 500ml three-necked flask, stirred at room temperature for 5 minutes, then 50ml of concentrated hydrochloric acid was added to the mixture, and 87.47 grams (0.8mol) of KSCN was added, stirred for 10min, then heated to 95°C for reflux reaction for 18h. As the temperature increases, the color of the system turns from red to orange, and finally turns to yellow. Cool the reflux system in an ice bath, filter with suction, and 2 O wash, followed by one wash with 95% ethanol. The obtained yellow crude product was recrystallized from ethanol, and then suction-filtered to obtain 53.13 g of a white powdery solid, with a yield of 81%.
[0052] Step two:
[0053] Put 100g of N-ethyl-N-phenylthiourea in a 500ml three-necked flask with 75ml of CHCl 3 Dissolve, then add 35ml CHCl of elemental bromine dropwise with a constant pressure dropping funnel 3 (30ml) mixed solution, control the temp...
Embodiment 3
[0061] step one:
[0062] Mix 48.47 g (0.4 mol) of N-ethylaniline with 60 ml H 2 O was added to a 500ml three-necked flask, stirred at room temperature for 5 minutes, then 40ml of concentrated hydrochloric acid was added to the mixture, and 54.67 grams (0.5mol) of KSCN was added, stirred for 10min, then heated to 95°C for reflux reaction for 18h. As the temperature increases, the color of the system turns from red to orange, and finally turns to yellow. Cool the reflux system in an ice bath, filter with suction, and 2 O wash, followed by one wash with ethanol. The obtained yellow crude product was recrystallized from ethanol, and then suction-filtered to obtain 51.13 g of a white powdery solid, with a yield of 79%.
[0063] Step two:
[0064] Put 100g of N-ethyl-N-phenylthiourea in a 500ml three-necked flask with 75ml of CHCl 3 Dissolve, then add 40ml CHCl of elemental bromine dropwise with a constant pressure dropping funnel 3 (40ml) mixed solution, control the temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com