Method for preparing octylene-1 by ethylene tetramerization reaction
A technology for tetramerization and reaction of ethylene, applied in chemical instruments and methods, hydrocarbons, organic compounds/hydrides/coordination complex catalysts, etc. low sex issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of (diphenyl) phosphorus nitrogen (isopropyl) phosphorus (diphenyl) ligand (C 27 h 27 NP 2 )
[0040] (1) Preparation of N, N-diisopropyl dichlorophosphoramide
[0041] in the N 2 Add dehydrated toluene (100mL) into a fully replaced stirred 250mL reactor, add PCl 3 (21.87mL, 0.25mol), and cooled to -20°C. Diisopropylamine (70 mL, 0.5 mol) was slowly added while stirring at room temperature, stirred for 3 hours, raised to room temperature and continued to react for 2 hours, then filtered and dried to obtain 38.1 g (0.19 mol, 74%) of the product.
[0042] (2) Preparation of phenylmagnesium bromide lattice reagent
[0043] in the N 2 Add dehydrated THF (100 mL) and magnesium powder (9.11 g, 0.375 mol) into a fully replaced stirred 250 mL reactor, cool down in an ice bath and slowly add bromobenzene (11.775 g, 0.075 mol) dropwise. After 2 hours, heat to reflux and continue the reaction for 2 hours to obtain the lattice reagent.
[0044] (3) Preparati...
Embodiment 2
[0055] 1. Preparation of (diphenyl) phosphorus nitrogen (isopropyl) phosphorus (diphenyl) ligand (C 27 h 27 NP 2 ) with embodiment 1.
[0056] 2. Preparation of modified active agent
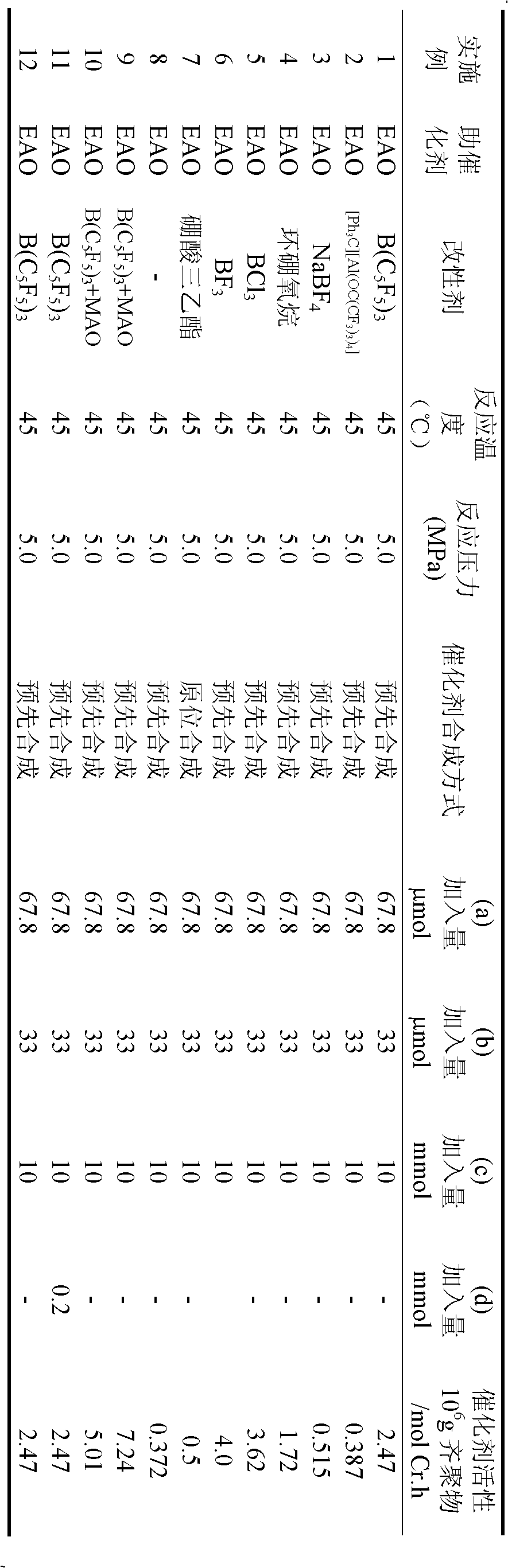
[0057] At -78°C, under vigorous stirring, slowly add 0.81 mL of twice distilled water (45 mmol) dropwise into 6.84 mL (50 mmol) of AlEt 3 In toluene (50mL) solution, after the dropwise addition, the system was naturally returned to room temperature, and the reaction was continued for 12h to obtain a toluene solution of EAO. [Ph 3 C][Al(OC(CF 3 ) 3 ) 4 ] (10mmol) of toluene solution 50mL, kept at room temperature for 6 hours after the dropwise addition, to obtain a modified activator.
[0058] 3. Preparation of catalyst
[0059] With embodiment 1. The modified activator added is the modified activator prepared in this example.
[0060] 4. Ethylene oligomerization
[0061] With embodiment 1. Obtain 6.38g of oligomerization product, catalyst activity is 3.87×10 5 g oligomer / mol Cr.h. ...
Embodiment 3
[0063] 1. Preparation of (diphenyl) phosphorus nitrogen (isopropyl) phosphorus (diphenyl) ligand (C 27 h 27 NP 2 ) with embodiment 1.
[0064] 2. Preparation of modified active agent
[0065] With embodiment 1. The difference is that the added modifier containing at least two heteroatoms is sodium tetrafluoroborate compound.
[0066] 3. Preparation of catalyst
[0067] With embodiment 1.
[0068] 4. Ethylene oligomerization
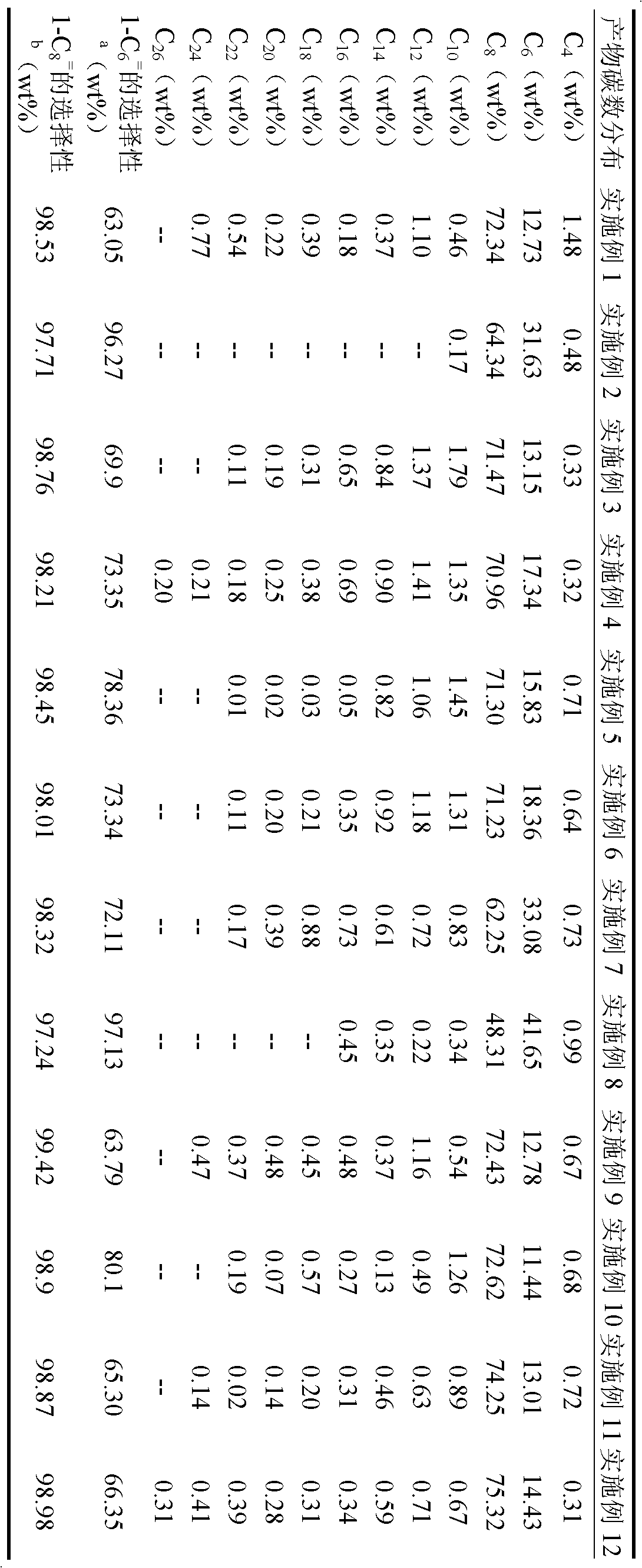
[0069] With embodiment 1. Obtain 8.5g of oligomerization product, catalyst activity is 5.15×10 5 g oligomer / molCr.h. The distribution of the oligomerization products is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com