Precursor suspension of lyotropic liquid crystal and preparation method thereof
A lyotropic liquid crystal and suspension technology, applied in the directions of liquid delivery, emulsion delivery, drug combination, etc., can solve the problems of weak gel strength, insufficient adhesion, and inability to guarantee retention, and achieve slow release and stability. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The precursor suspension of embodiment 1 lyotropic liquid crystal
[0037] Contains the following components:
[0038]
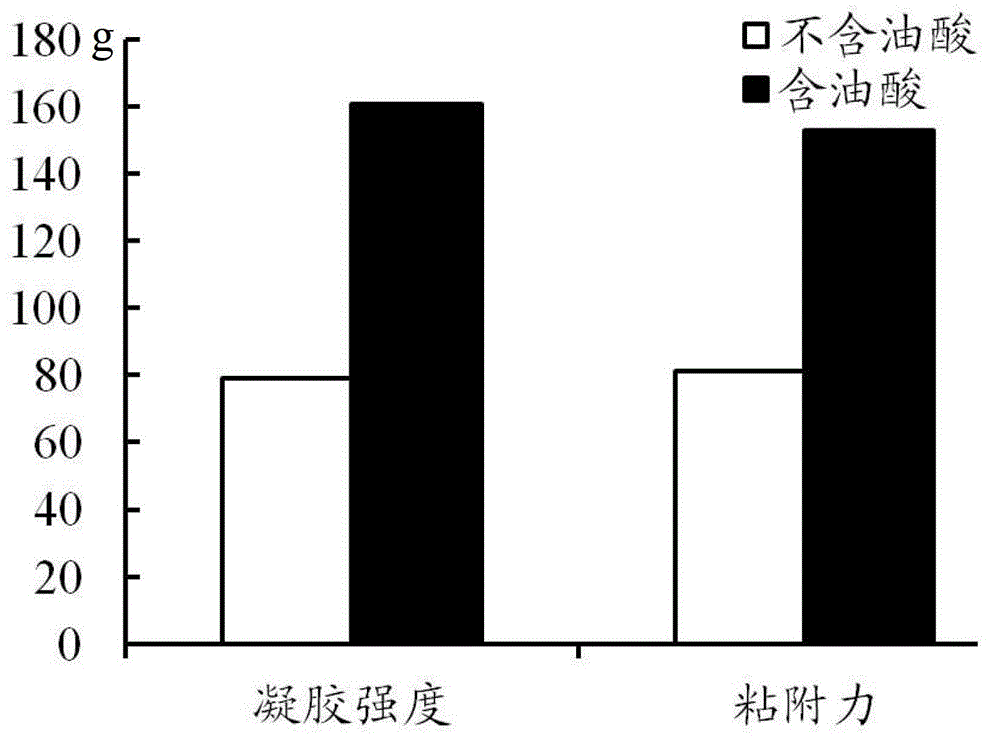
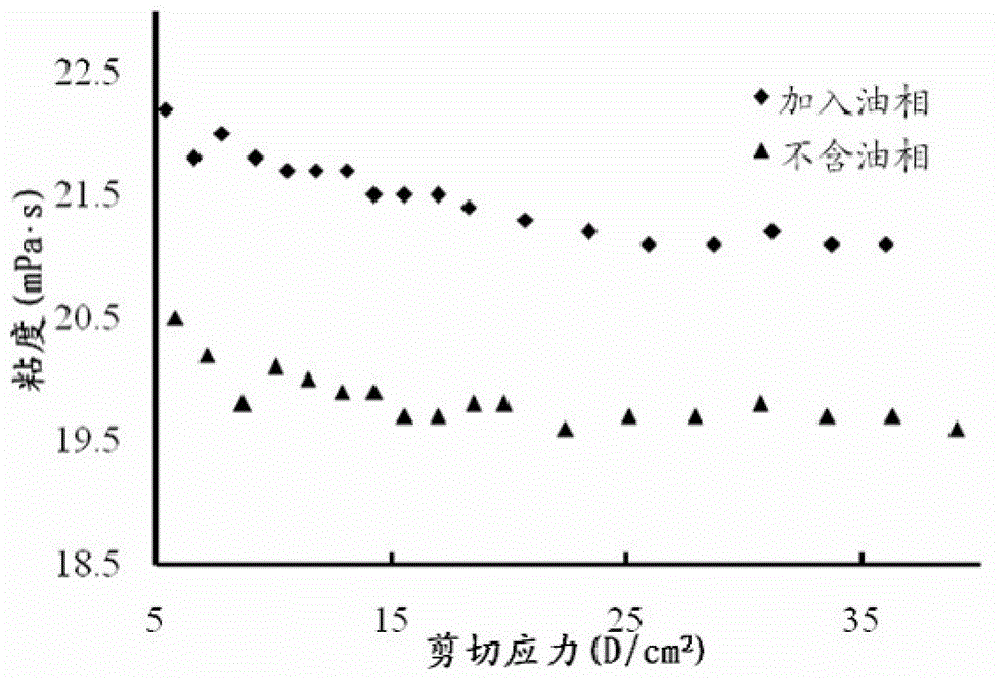
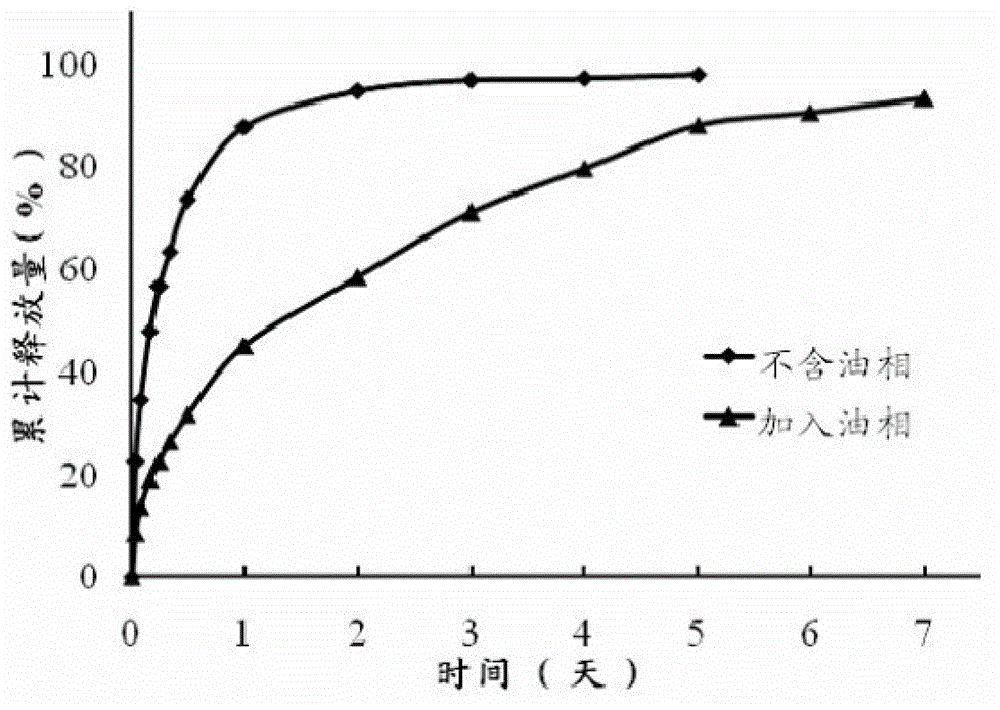
[0039] The preparation method of the precursor suspension of this embodiment is: after mixing glycerol monooleate, oleic acid and 2-pyrrolidone evenly, add micronized doxycycline hydrochloride (1 ~10μm) raw material drug, after mixing evenly, it is ready. Due to the poor stability of doxycycline hydrochloride, it should be used immediately after mixing. The drug loading of the precursor suspension in this embodiment is 10%, and the drug can be released in vitro for up to one week. The strength of the formed lyotropic liquid crystal gel is about 160 g, and the adhesion is 150 g.
Embodiment 2
[0040] The precursor suspension of embodiment 2 lyotropic liquid crystal
[0041] Contains the following components:
[0042]
[0043] The preparation method of the precursor suspension in this example is: dissolving the doxycycline hydrochloride bulk drug in N-methylpyrrolidone to form a uniform and transparent solution, adding glycerol monooleate at a stirring speed of 2500r / min Esters, oleic acid, and drugs are slowly precipitated during the addition of the two, forming smaller particles (1-10 μm) suspended in the entire system, that is. Due to the poor stability of doxycycline hydrochloride, it should be used immediately after mixing. The precursor suspension in this embodiment is released externally for 7-10 days, and the formed lyotropic liquid crystal gel has a strength of about 80 g and an adhesive force of about 20 g. Compared with Example 1, the weight ratio of solute liquid crystal material is reduced, accompanied by the reduction of gel strength and adhesion. ...
Embodiment 3
[0044] The precursor suspension of embodiment 3 lyotropic liquid crystal
[0045] Contains the following components:
[0046]
[0047] The preparation method of the precursor suspension in this embodiment is as follows: after mixing glycerol monooleate, propylene glycol, and soybean oil evenly, add micronized metronidazole raw drug powder (1-10 μm), and mix evenly to obtain . The drug loading amount of the precursor suspension in this embodiment is 25%, the content is uniform, and the drug release in vitro is 2-3 days. The gel strength is less than 80g, and the adhesion is about 20g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com