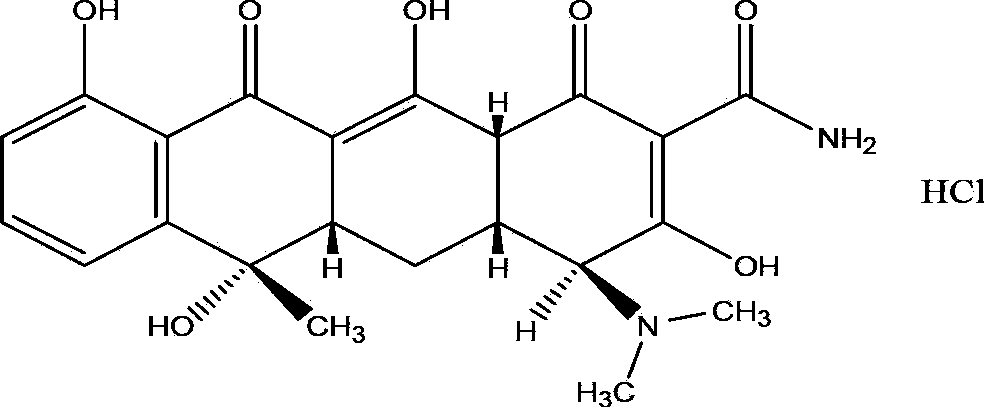
Method for preparing high-purity tetracycline hydrochloride
A technology of tetracycline hydrochloride and tetracycline, which is applied in the preparation of carboxylic acid amide, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of poor quality of crystallized products, achieve quality improvement, optimize temperature control process, and no coalescence Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
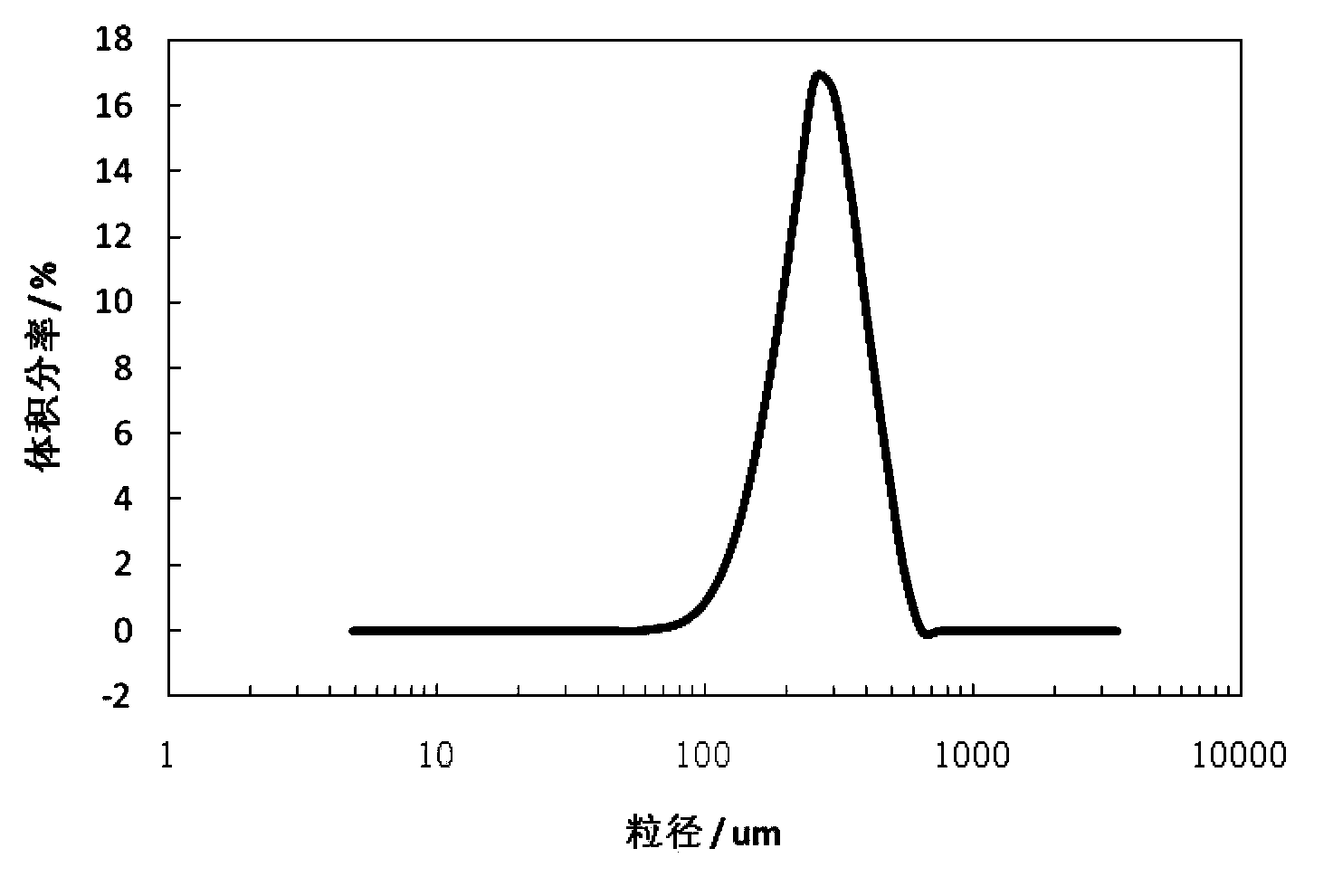
[0024] Dissolve 30g of tetracycline urea double salt (0.0624 mol) in 150ml of n-butanol and 100ml of methanol mixed solvent, stir at 10°C for 5 minutes, add 6.1ml of concentrated hydrochloric acid (HCl 0.0626 mol) with a mass concentration of 37.5%, and continue stirring After filtering. Move the filtrate into the crystallizer, add 1.0g of seed crystals at a heating rate of 2°C / min to 30°C, continue to maintain the same heating rate until 40°C, maintain constant temperature for 30 minutes, and then cool down at a rate of 3°C / min The rate was reduced to 20 °C. The crystalline product is filtered, washed with acetone, and dried at 40°C for 12 hours under a vacuum of 0.05 MPa. The final crystalline product has a purity of 98.3% and a main particle size of 210 μm. The product quality meets the standards of the Chinese Pharmacopoeia 2000 edition. The scanning electron micrographs of the crystalline products are as follows figure 1 As shown, the product particle size distribution ...
Embodiment 2
[0026] Dissolve 30g of tetracycline urea double salt (0.0624 mol) in 270ml of n-butanol and 30ml of methanol mixed solvent, stir at 15°C for 5 minutes, add 12.3ml of 30% concentrated hydrochloric acid (HCl 0.101 mol), and continue stirring After filtering. Move the filtrate into the crystallizer, add 0.3g of seed crystals at a heating rate of 8°C / min to 32°C, continue to maintain the same heating rate until 50°C, maintain constant temperature for 20 minutes, and then cool down at a rate of 5°C / min The rate was reduced to 15 °C. The crystalline product is filtered, washed with acetone, and dried for 4 hours at 45°C under a vacuum of 0.1 MPa. The purity of the final crystalline product is 98.1%, and the main particle size is 205 μm. The product quality meets the standards of the Chinese Pharmacopoeia 2000 edition.
Embodiment 3
[0028] Dissolve 30g of tetracycline urea double salt (0.0624 mol) in 150ml of n-butanol and 60ml of ethanol mixed solvent, stir at 15°C for 8 minutes, add 7.3ml of concentrated hydrochloric acid (HCl 0.072 mol) with a mass concentration of 36%, and continue stirring filter. Move the filtrate into the crystallizer, add 0.6g of seed crystals at a heating rate of 1°C / min to 27°C, continue to maintain the same heating rate until 48°C, maintain constant temperature for 40 minutes, and then cool down at a rate of 4°C / min The rate was reduced to 10 °C. The crystalline product is filtered, washed with acetone, and dried at 50°C for 8 hours under a vacuum of 0.06 MPa. The final crystalline product has a purity of 98.2% and a main particle size of 220 μm. The product quality meets the standards of the Chinese Pharmacopoeia 2000 edition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com