Asarone injection and preparation method thereof
A Xinnao injection and asarone technology, which is applied in the fields of medical formula, digestive system, respiratory system diseases, etc., can solve the problems of lysophospholipid side effects and high production cost, and achieve high solubilizing ability, convenient clinical use, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
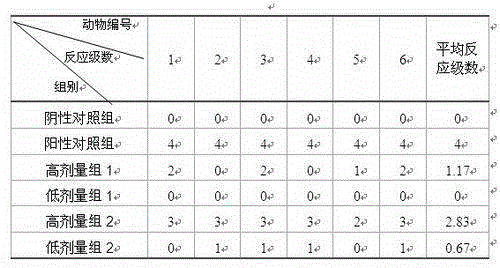
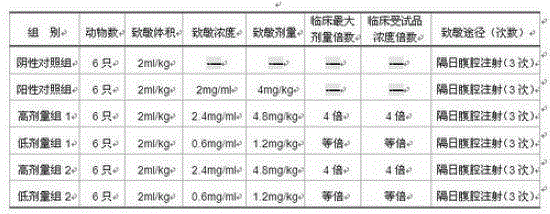
Image
Examples
Embodiment 1
[0032] Example 1 (1 part by weight of α-asarone, 8 parts of HS15, 50 parts of propylene glycol, and 12 parts of ethanol)
[0033] Weigh 4g α-asarone, 40g HS15, 200g propylene glycol, seal for use; measure 60ml of ethanol and seal for use; prepare 0.01mol / L hydrochloric acid solution with concentrated hydrochloric acid, seal for use; measure 500ml of water for injection, 60 Add 32g HS15 in a water bath at ℃, stir to dissolve; add 4g α-asarone in a water bath at 60℃, stir to dissolve, let cool to room temperature; add 200g propylene glycol and 60ml ethanol in turn, stir well; add water for injection to 950ml, Stir and mix evenly; adjust the pH to 6.03 with 0.01mol / L hydrochloric acid solution, add water for injection to 1000ml, mix evenly, first filter through a 0.45μm microporous membrane, then filter through a 0.22μm microporous membrane, and pack in Put it in a 2ml ampoule, melt seal it, and sterilize it at 100°C for 30 minutes to obtain α-asarone injection.
Embodiment 2
[0034] Example 2 (1 part by weight of α-asarone, 10 parts of HS15, 50 parts of propylene glycol, and 12 parts of ethanol)
[0035]Weigh 4g α-asarone, 40g HS15, 200g propylene glycol, seal for use; measure 60ml of ethanol and seal for use; prepare 0.01mol / L hydrochloric acid solution with concentrated hydrochloric acid, seal for use; measure 500ml of water for injection, 60 Add 40g HS15 in a water bath at ℃, stir to dissolve; add 4g α-asarone in a water bath at 60℃, stir to dissolve, let cool to room temperature; add 200g propylene glycol and 60ml ethanol in turn, stir well; add water for injection to 950ml, Stir and mix evenly; adjust the pH to 6.08 with 0.01mol / L hydrochloric acid solution, add water for injection to 1000ml, mix evenly, first filter through a 0.45μm microporous membrane, then filter through a 0.22μm microporous membrane, and pack in Put it in a 2ml ampoule, melt seal it, and sterilize it at 100°C for 30 minutes to obtain α-asarone injection.
Embodiment 3
[0036] Example 3 (1 part of α-asarone based on parts by weight, 12 parts of HS15, 50 parts of propylene glycol, and 12 parts of ethanol)
[0037] Weigh 4g α-asarone, 40g HS15, 200g propylene glycol, seal for use; measure 60ml of ethanol and seal for use; prepare 0.01mol / L hydrochloric acid solution with concentrated hydrochloric acid, seal for use; measure 500ml of water for injection, 60 Add 48g HS15 in a water bath at ℃, stir to dissolve; add 4g α-asarone in a water bath at 60℃, stir to dissolve, let cool to room temperature; add 200g propylene glycol and 60ml ethanol in turn, stir well; add water for injection to 950ml, Stir and mix evenly; adjust the pH to 6.10 with 0.01mol / L hydrochloric acid solution, add water for injection to 1000ml, mix evenly, first filter through a 0.45μm microporous membrane, then filter through a 0.22μm microporous membrane, and pack in Put it in a 2ml ampoule, melt seal it, and sterilize it at 100°C for 30 minutes to obtain α-asarone injection....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com