Compounds with MEK inhibitory function and their preparation methods and applications
A compound, coumarin technology, applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve problems such as large toxic and side effects, low kinase selectivity, and inability to produce specific effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
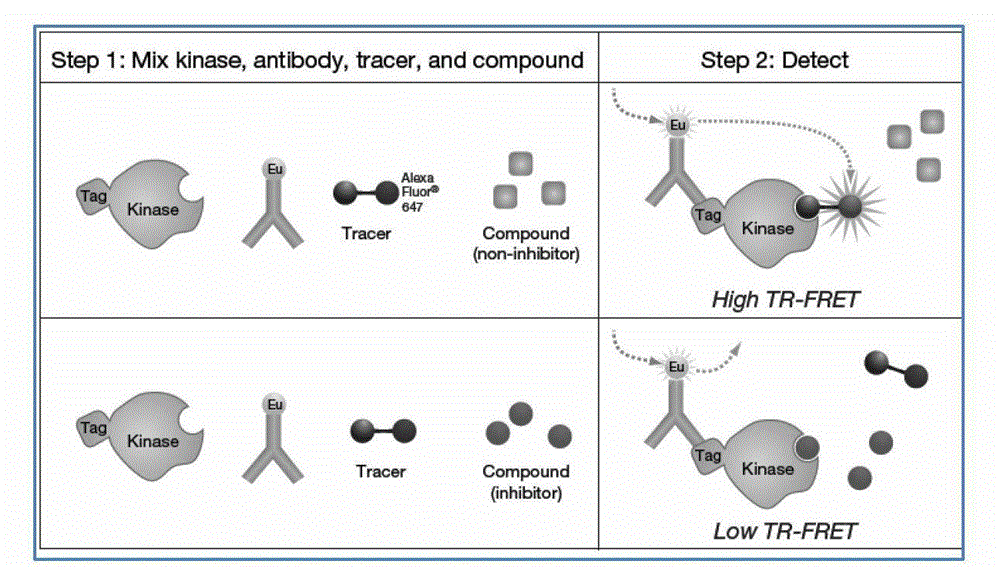
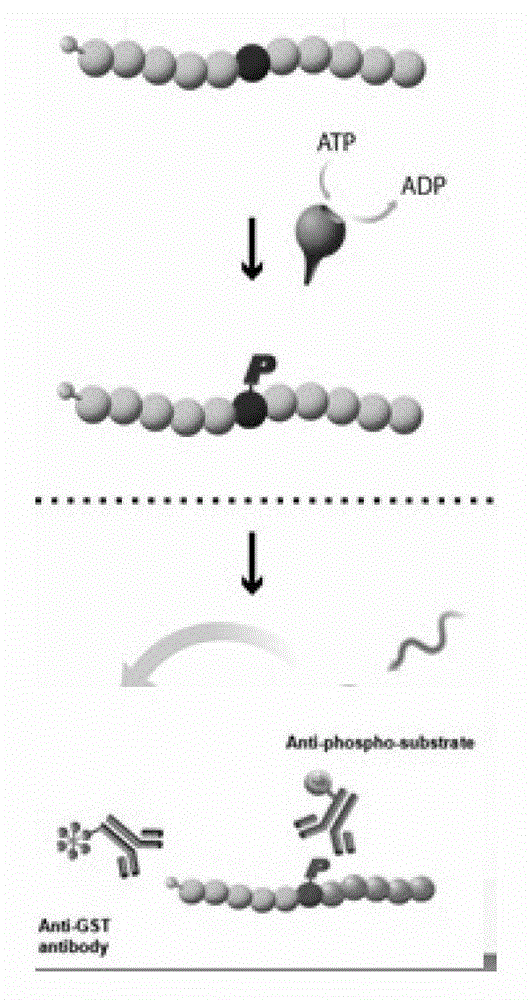
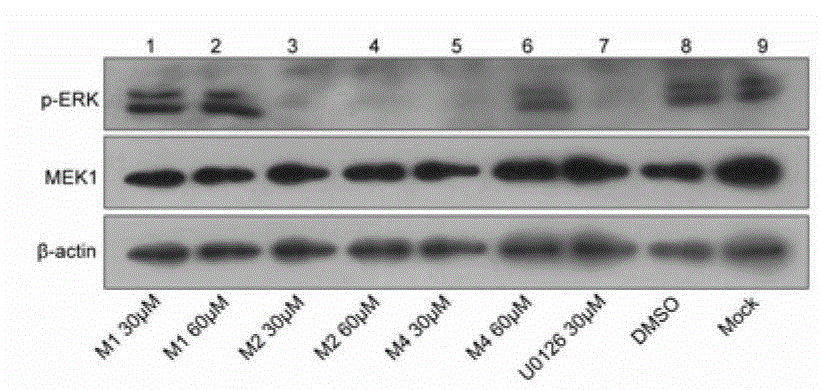
Image
Examples
Embodiment 1
[0070] The synthesis of embodiment 1,3-benzyl-4-methyl-7-dimethylcarbamate base coumarin (compound M2)
[0071] first step
[0072]Slowly add metal sodium (50mmol, 1.15g) into 20ml of absolute ethanol to form sodium alkoxide, add 50mmol of ethyl acetoacetate under stirring at 80°C, and continue stirring for 10 minutes; Bian dehydrated ethanol solution, heated to reflux until the reactant is almost neutral. Cool, filter to remove solid sodium bromide, dry the filtrate over anhydrous magnesium sulfate, and separate by column chromatography (petroleum ether: ethyl acetate = 50:1) (v / v) to obtain ethyl 3-benzyl acetoacetate, a yellow oily liquid 7.17 g, yield 65.1%. 1 HNMR (300MHz, CDCl 3 ):δ1.16-1.21(t,J=7.1Hz,3H,CH 3 ),2.17(s,1H,CH 3 ), 3.14-3.16 (d, J=7.5Hz, 2H, PhCH 2 ),3.76-3.81(t,J=7.5Hz,1H,CH),4.09-4.17(d,J=7.2Hz,2H,C H 2 CH 3 ), 7.16-7.28 (m, 5H, PhH).
[0073] second step
[0074] Resorcinol (5mmol, 0.55g) and ethyl 3-benzyl acetoacetate (5mmol, 1.10g) were dis...
Embodiment 2
[0077] The synthesis of embodiment 2,3-benzyl-4-methyl-6-chloro-7-dimethylcarbamate base coumarin (compound M4)
[0078] first step
[0079] 4-chlororesorcinol (5mmol, 0.72g) and ethyl 3-benzyl acetoacetate (5mmol, 1.10g) were dissolved in 11ml of 70% sulfuric acid and reacted at room temperature for 24h. Ice water was added to the mixture solution to precipitate a solid, which was filtered and washed with water to obtain a crude product. The crude product was recrystallized from ethanol to obtain 0.652 g of the product 3-benzyl-4-methyl-6-chloro-7-hydroxycoumarin (compound M3), a white powder, yield 43.5%, mp: 261-263°C. 1 HNMR(400MHz,DMSO):δ2.40(s,3H,CH 3 ),3.93(s,2H,CH 2 ), 6.89 (s, 1H, PhH), 7.15-7.28 (m, 5H, PhH), 7.79 (s, 1H, PhH), 11.26 (s, 1H, OH).
[0080] second step
[0081] 3-Benzyl-4-methyl-6-chloro-7-hydroxycoumarin (1mmol, 0.30g) was dissolved in 12ml of DMF, and sodium hydride (1.25mmol, 0.038g) was added under nitrogen protection, and reacted at room temp...
Embodiment 3
[0082] Embodiment 3, the synthesis of 3-p-fluorobenzyl-4-methyl-7-dimethylcarbamate base coumarin (compound M5)
[0083] first step
[0084] Add ethyl acetoacetate (10mmol, 1.26ml) to 3.76ml (10mmol) 21% sodium ethoxide solution, reflux reaction at 80°C, when the reactant slightly boils, add dropwise the dehydrated ethanol solution of 4-fluorobromide, continue React until the solution is almost neutral. Cool, remove solid sodium bromide by filtration, dry the filtrate over anhydrous magnesium sulfate, and separate by column chromatography (petroleum ether: ethyl acetate = 50:1) (v / v) to obtain 1.41 g of ethyl 3-fluorobenzyl acetoacetate , yellow oily liquid, yield 59%.
[0085] 1 HNMR (400MHz, CDCl 3 ):δ1.18-1.23(t,J=7.2Hz,3H,CH 2 C H 3 ),2.12(s,3H,C H 3 CO),3.11-3.14(dd,J=1.2,7.2Hz,2H,CH 2 Ph),3.70-3.75(t,J=7.6Hz,1H,CH),4.12-4.16(ddd,J=1.2,7.2,11.6Hz,2H,C H 2 CH 3 ), 6.93-6.98 (m, 2H, PhH), 7.12-7.16 (m, 2H, PhH).
[0086] second step
[0087] Dissolve resorcin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com