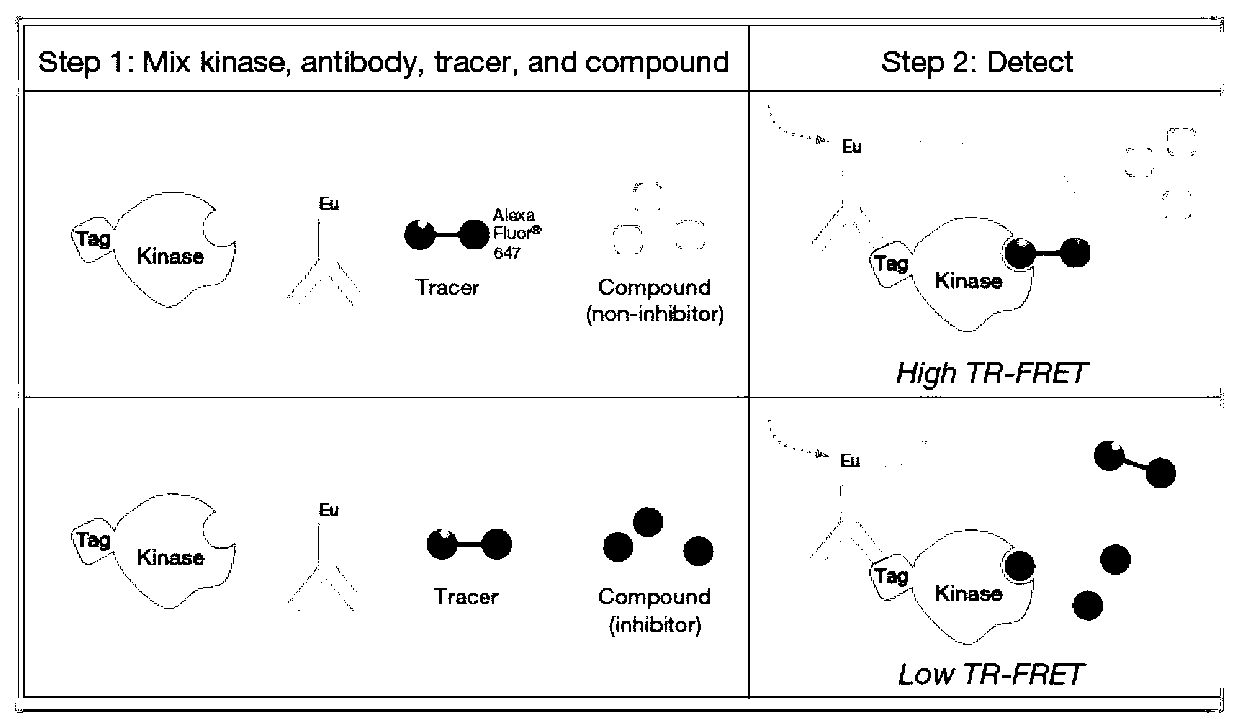
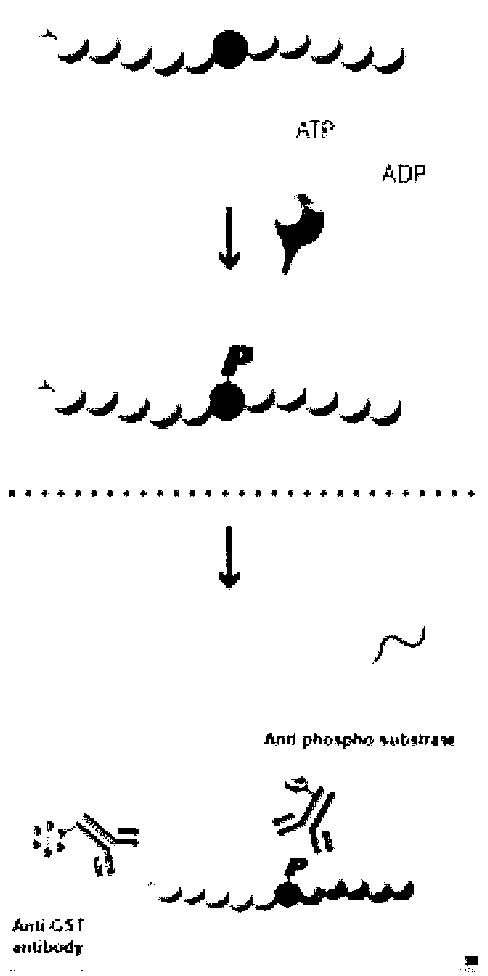
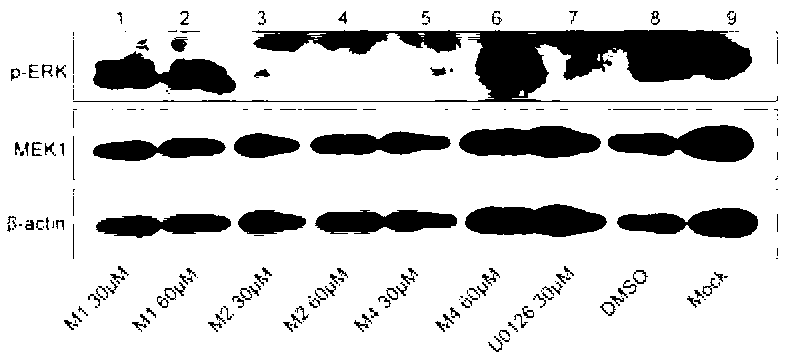
Compound with MEK (Mitogen-activated and Extracellular signal-regulated Kinase) inhibiting function as well as preparation method and application of compound
A compound and alkyl technology, applied in the field of compounds with MEK inhibitory function and their preparation, can solve the problems of low kinase selectivity, large toxic and side effects, and inability to produce specific effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1. Synthesis of 3-benzyl-4-methyl-7-dimethylcarbamate coumarin (Compound M2)
[0071] first step
[0072] Slowly add sodium metal (50mmol, 1.15g) to 20ml of absolute ethanol. After sodium alkoxide is formed, add 50mmol of ethyl acetoacetate under stirring at 80°C and continue stirring for 10 minutes; when the reactant is boiled and refluxed, add 55mmol of bromine dropwise Bian anhydrous ethanol solution, heated to reflux until the reactants are almost neutral. Cool, filter to remove solid sodium bromide, dry the filtrate with anhydrous magnesium sulfate, and separate by column chromatography (petroleum ether: ethyl acetate = 50:1) (v / v) to obtain ethyl 3-benzyl acetoacetate, a yellow oily liquid 7.17g, the yield was 65.1%. 1 H NMR(300MHz, CDCl 3 ):δ1.16-1.21(t,J=7.1Hz,3H,CH 3 ), 2.17(s,1H,CH 3 ),3.14-3.16(d,J=7.5Hz,2H,PhCH 2 ), 3.76-3.81(t,J=7.5Hz,1H,CH),4.09-4.17(d,J=7.2Hz,2H,C H 2 CH 3 ), 7.16-7.28 (m, 5H, PhH).
[0073] Second step
[0074] Dissolve resorcinol (5mm...
Embodiment 2
[0077] Example 2. Synthesis of 3-benzyl-4-methyl-6-chloro-7-dimethylcarbamate coumarin (Compound M4)
[0078] first step
[0079] Dissolve 4-chlororesorcinol (5mmol, 0.72g) and ethyl 3-benzylacetoacetate (5mmol, 1.10g) in 11ml of 70% sulfuric acid, and react at room temperature for 24h. Ice water was added to the mixture solution to precipitate a solid, which was filtered and washed with water to obtain a crude product. The crude product was recrystallized with ethanol to obtain 0.652 g of 3-benzyl-4-methyl-6-chloro-7-hydroxycoumarin (compound M3), a white powder, with a yield of 43.5%, mp: 261-263°C. 1 HNMR(400MHz,DMSO):δ2.40(s,3H,CH 3 ),3.93(s,2H,CH 2 ), 6.89 (s, 1H, PhH), 7.15-7.28 (m, 5H, PhH), 7.79 (s, 1H, PhH), 11.26 (s, 1H, OH).
[0080] Second step
[0081] 3-benzyl-4-methyl-6-chloro-7-hydroxycoumarin (1mmol, 0.30g) was dissolved in 12ml DMF, sodium hydride (1.25mmol, 0.038g) was added under nitrogen protection, and reacted at room temperature for 30 minutes , Add N,N-dimeth...
Embodiment 3
[0082] Example 3. Synthesis of 3-p-fluorobenzyl-4-methyl-7-dimethylcarbamate coumarin (Compound M5)
[0083] first step
[0084] Add ethyl acetoacetate (10mmol, 1.26ml) to 3.76ml (10mmol) 21% sodium ethoxide solution, and reflux at 80℃ for reaction. When the reactant is slightly boiling, add 4-fluorobromide ethanol solution dropwise, continue React until the solution is almost neutral. Cool, filter to remove solid sodium bromide, dry the filtrate with anhydrous magnesium sulfate, and separate by column chromatography (petroleum ether: ethyl acetate = 50:1) (v / v) to obtain 1.41 g of ethyl 3-p-fluorobenzyl acetoacetate , Yellow oily liquid, yield 59%.
[0085] 1 H NMR(400MHz, CDCl 3 ):δ1.18-1.23(t,J=7.2Hz,3H,CH 2 C H 3 ), 2.12(s,3H,C H 3 CO),3.11-3.14(dd,J=1.2,7.2Hz,2H,CH 2 Ph),3.70-3.75(t,J=7.6Hz,1H,CH),4.12-4.16(ddd,J=1.2,7.2,11.6Hz,2H,C H 2 CH 3 ), 6.93-6.98 (m, 2H, PhH), 7.12-7.16 (m, 2H, PhH).
[0086] Second step
[0087] Dissolve resorcinol (3.43mmol, 0.377g) and ethyl 3-p-fluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com