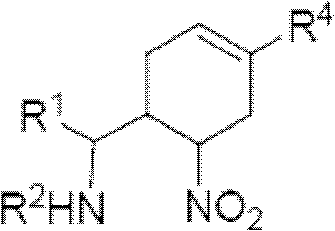
Cyclohexene compound having influenza virus neuraminidase inhibition activity, preparation method and application
A compound and synthesis method technology, applied in the field of new cyclohexene compounds, can solve problems such as no cyclohexene compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0088] Another specific embodiment provides an intermediate compound with general formula 5 for preparing the compound of general formula I:
[0089]
[0090] Formula 5
[0091] or pharmaceutically acceptable salts thereof, and resolved enantiomers and purified diastereomers, wherein:
[0092] R 1 Can be H or C 1-12 alkyl;
[0093] Boc may be t-butoxycarbonyl; and
[0094] R 4 Can be -(CH 2 ) n CO 2 H, -(CH 2 ) n P(O)(OH) 2 ,-(CH 2 ) n CO 2 R 1a , or -(CH 2 ) n P(O)(OR 1a ) 2 , where R 1a Can be H or C 1-6 Alkyl, n is an integer from 0 to 4, or a salt of the above groups. Another specific embodiment of the present invention provides intermediate compounds with general formula 6 for the preparation of compounds of general formula I:
[0095]
[0096] Formula 6
[0097] or pharmaceutically acceptable salts, and resolved enantiomers and purified diastereomers, wherein
[0098] R 1 Can be H or C 1-12 alkyl;
[0099] R 2 Can be H, -C(O)R 1a , -C(O)O...
Embodiment 1
[0195] Preparation of compound (4S, 5S)-4-((S)-1-acetamido-3-methylbutyl)-5-amino-1-cyclohexenecarboxylic acid
[0196]
[0197] Step A: (S,E)-6-Methyl-4-tert-butoxycarbonylamino-2-heptenal
[0198]
[0199] L-leucinol (1.29 g, 11.0 mmol) was dissolved in methanol (50 ml), and Boc anhydride (or di-tert-butyl dicarbonate, 2.64 g, 12.1 mmol) in methanol ( 10 ml) solution, stirred at room temperature for 14 hours. Rotary evaporation under reduced pressure until the solvent was evaporated to dryness, dichloromethane (30 ml) was added and washed with saturated sodium bicarbonate solution (3×20 ml), the organic phases were combined and dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to give Color transparent liquid (2.27 g). Under nitrogen protection, oxalyl chloride (2.00 g, 15.7 mmol) was dissolved in dichloromethane (25 ml), and after cooling to -78 ° C, DMSO (2.45 g, 31.4 mmol) was added dropwise in dichloromethane (5 mL) solution...
Embodiment 2
[0213] Preparation of compound (4R, 5R)-4-((S)-1-acetamido-3-methylbutyl)-5-amino-1-cyclohexene diammonium phosphonate
[0214]
[0215] Step A: (S,E)-6-Methyl-4-tert-butoxycarbonylamino-2-heptenal
[0216]
[0217] The title compound was prepared according to the procedure described in Example 1, Step A.
[0218] Step B: Tetraethyl 3-nitropropyl-1,1-diphosphonate
[0219]
[0220] Tetraethyl methylene diphosphonate (11.7 g, 40.7 mmol) was dissolved in anhydrous methanol (100 mL), and paraformaldehyde (6.1 g, 203.5 mmol) and diethylamine (4.2 mL, 40.7 mmol), heated to reflux for 24 hours, and the solvent was evaporated to dryness by rotary evaporation under reduced pressure to obtain 12.3 grams of yellow liquid. The yellow liquid (12.3 g) was dissolved in toluene (100 ml), p-toluenesulfonic acid (344 mg, 2.0 mmol) was added, and heated to reflux in a water trap for 24 hours. The solvent was removed under reduced pressure, and the fraction with a boiling point of 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com