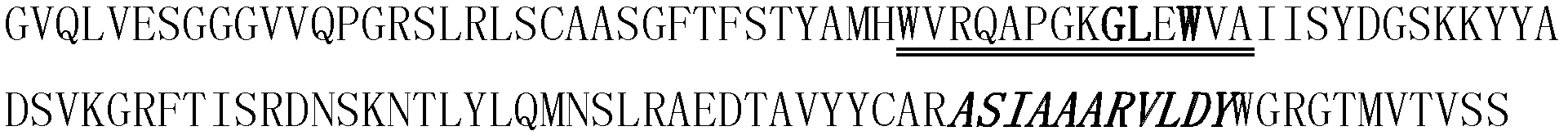Human Vascular Endothelial Growth Factor Antigen Epitope and Epitope Vaccine
A vascular endothelium and growth factor technology, applied in anti-tumor drugs, antibody medical components, peptide/protein components, etc., can solve the problems of high treatment cost and antibody heterogeneity, and achieve low clinical use and good immunogenicity. , the effect of low clinical treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Analysis and verification of human VEGF epitope
[0059] Protein epitope analysis methods generally include synthetic peptide verification method, computer-aided program prediction method, and protein three-dimensional structure analysis method, but any method has limitations, and the antigen epitope prediction based on protein three-dimensional structure is accurate. The highest degree. At present, two or more analysis methods are generally combined to predict the epitope of a protein, and finally to determine whether an epitope is a real epitope must be verified by experiments.
[0060] The present invention predicts the most likely human VEGF antigen epitope by using computer-aided program prediction method and protein three-dimensional structure analysis method, and then verifies whether the epitope is the real antigen epitope through experiment.
[0061] First, use the BepiPred (http: / / www.cbs.dtu.dk / services / BepiPred / ) epitope prediction program to init...
Embodiment 2
[0106] Example 2: Construction, expression and purification of human VEGF epitope vaccine expression vector
[0107] The human single domain antibody BT32 / A6 (Journal of Biology Chemistry 2001, 276: 24774-24780) was camelized, that is, the 9th, 10th and 12th amino acids of FR2 of the human single domain antibody BT32 / A6 Respectively replaced by glutamic acid, arginine and glycine, its sequence is shown in SEQ ID NO: 9, (see attached Figure 8 , wherein the double underlined part is the framework region 2 (FR2); the framed part is the amino acid after camelization transformation; the single underlined part is the CDR3 region).
[0108] Replace the appended with human VEGF epitope sequence "QIMRIKPHQGQHIGEM" Figure 8 The "DRLKVEYYDSSGYYVSRFGA" amino acid sequence of the CDR3 region in the middle, thus forming the amino acid sequence of a new protein, its amino acid sequence is shown in SEQ ID NO: 10, (as attached Figure 9 , wherein the double underlined part is the amino aci...
Embodiment 3
[0125] Embodiment 3: Analysis of immune effect of human VEGF epitope vaccine
[0126] Five female Balb / c mice aged 6-8 weeks were immunized with the human VEGF epitope vaccine obtained in Example 2, and the immune effect of the vaccine was observed.
[0127] The dose of immunization was 50 μg per mouse, the site of immunization was the abdominal cavity, and the immunization procedure was on the 0th, 14th, 21st and 35th days. Freund's complete adjuvant was used for the first immunization, Freund's incomplete adjuvant was used for the second and third immunization, and no adjuvant was added for the last immunization. Seven days after the last immunization, blood was collected to test the immune effect of the vaccine.
[0128] The pre-immune serum and post-immune serum of five animals were diluted 10,000 times, and then ELISA method was used to detect their recognition of human VEGF, and the coating concentration of human VEGF was 2 μg / ml. The test results showed that, after 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com