Doxorubicin bonding medicine and preparation method thereof
A doxorubicin-bonded drug technology, applied in the field of doxorubicin-bonded drug and its preparation, can solve the problems of cumbersome preparation process, too stable chemical bond, low drug loading, etc., and achieves enhanced drug effect and good biological phase. capacitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of doxorubicin-bonded drug, comprising the following steps:
[0043]Dextran, carboxylated doxorubicin derivatives, lactobionic acid with the structure of formula (V) and folic acid with the structure of formula (VI) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiazyme Amine·hydrochloride and 4-dimethylaminopyridine are reacted in an organic solvent to obtain a doxorubicin bonded drug, and the carboxylated doxorubicin derivative has formula (VII-a), formula (VII-b), Formula (VII-c) or formula (VII-d) structure:
[0044]
[0045]
[0046] In the present invention, dextran, carboxylated doxorubicin derivatives, lactobionic acid with a structure of formula (V) and folic acid with a structure of formula (VI) are first added to an organic solvent, and then 1-(3-dimethylamino Propyl)-3-ethylcarbodiimide·hydrochloride and 4-dimethylaminopyridine were reacted to obtain a doxorubicin-bonded drug. In the present inventi...
Embodiment 1
[0057] Put 580.0mg (0.001mol) of doxorubicin hydrochloride, 156.1mg (0.001mol) of cis-3-carboxyglutaconic anhydride and 101.2mg (0.001mol) of triethylamine in a dry reaction flask, and add 5mL Anhydrous N,N-dimethylformamide was dissolved, and reacted at 25°C for 24 hours under the condition of stirring with a stirrer. After the reaction was completed, pour the obtained reaction mixture into 100 mL of ethyl acetate to dilute, and dilute with saturated chloride Washing with aqueous sodium solution, drying, filtering and concentrating to obtain carboxylated doxorubicin derivatives.
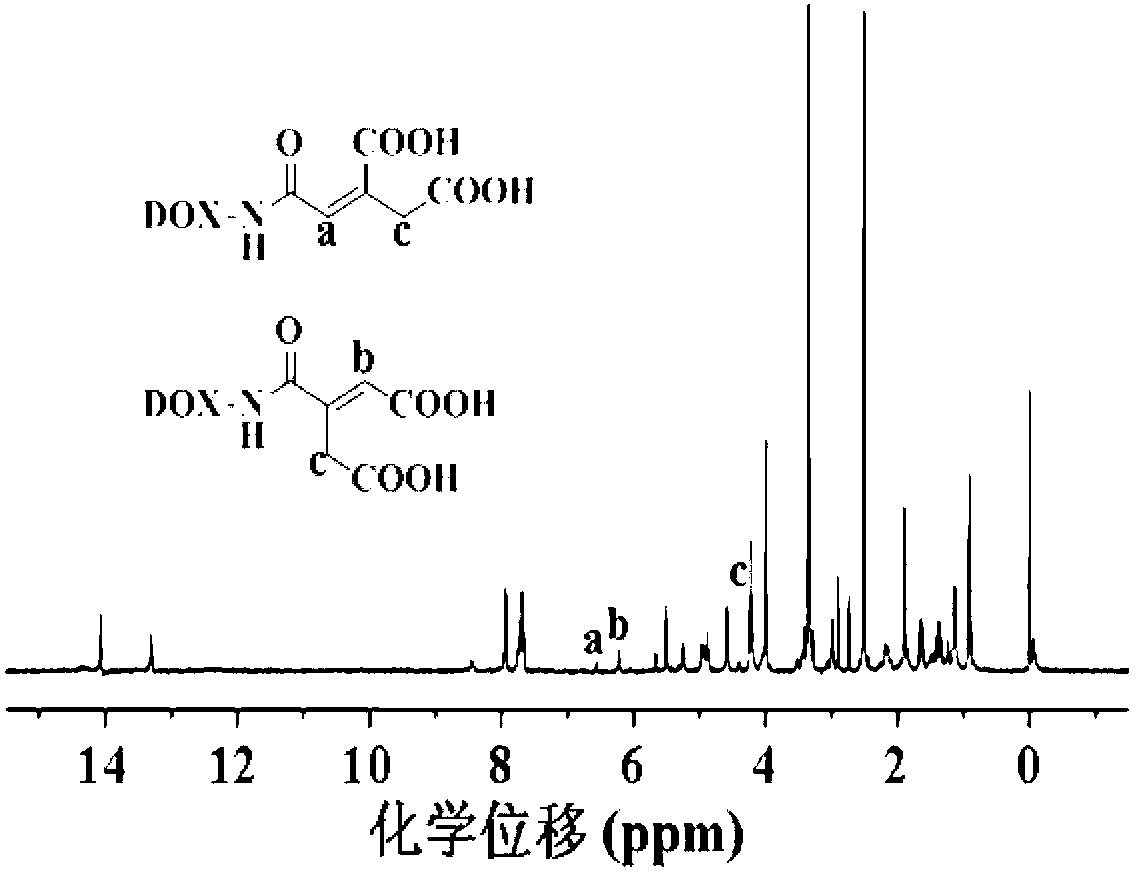
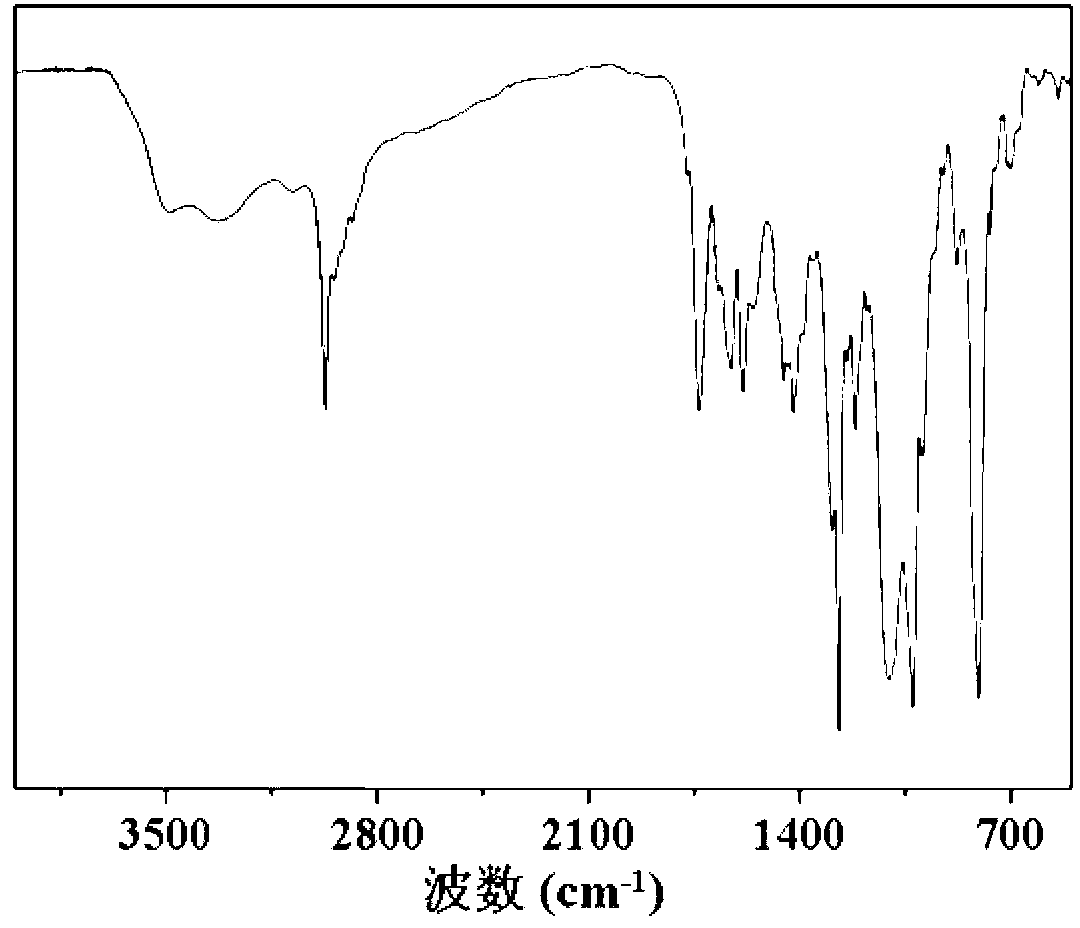
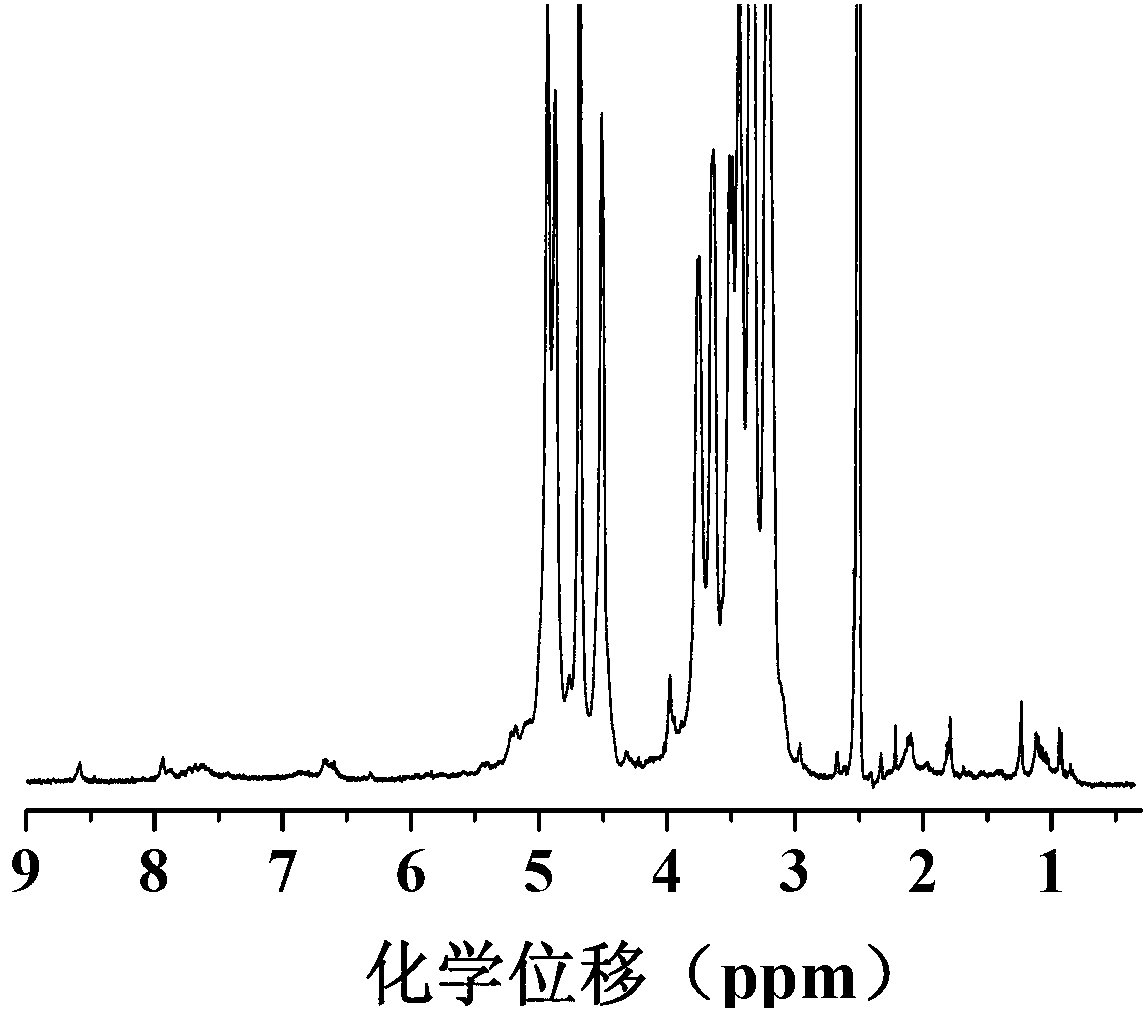
[0058] Carry out nuclear magnetic resonance analysis to the doxorubicin derivative of described carboxylation, the result sees figure 1 , figure 1 The hydrogen nuclear magnetic resonance spectrum of the carboxylated doxorubicin derivatives prepared for Example 1 of the present invention; the carboxylated doxorubicin derivatives were subjected to infrared analysis, and the results can be found in ...
Embodiment 2
[0060] Put 580.0mg (0.001mol) of doxorubicin hydrochloride, 152.2mg (0.001mol) of 1,2-dicarboxycyclohexene anhydride and 101.2mg (0.001mol) of triethylamine in a dry reaction bottle, add 5mL Anhydrous N,N-dimethylformamide was dissolved, and reacted at 25°C for 24 hours under the condition of stirring with a stirrer. After the reaction was completed, pour the obtained reaction mixture into 100 mL of ethyl acetate to dilute, and dilute with saturated chloride After washing with aqueous sodium solution, drying, filtering and concentrating, carboxylated doxorubicin derivatives are obtained.
[0061] The carboxylated doxorubicin derivatives were subjected to nuclear magnetic resonance analysis and infrared analysis, and the results showed that the carboxylated doxorubicin derivatives prepared in Example 2 of the present invention had the structure of formula (II-a).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com