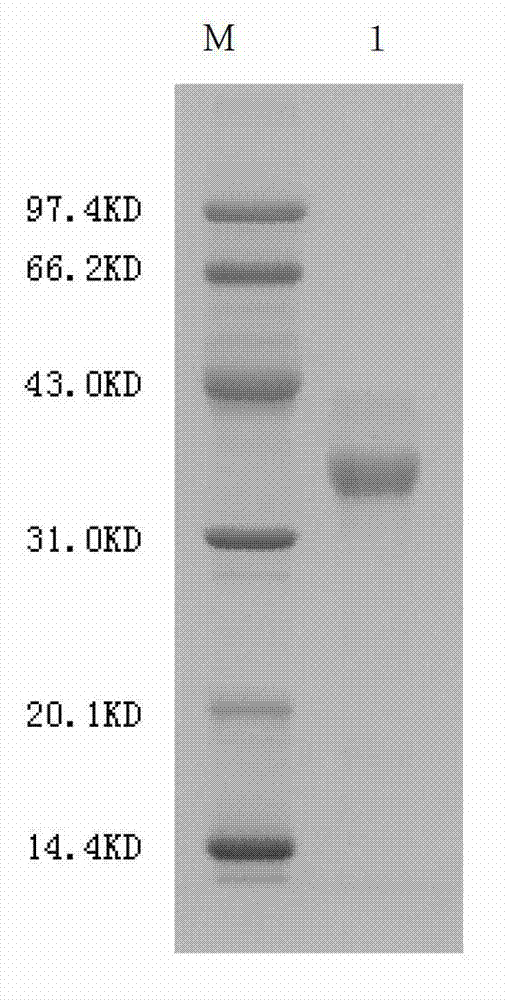
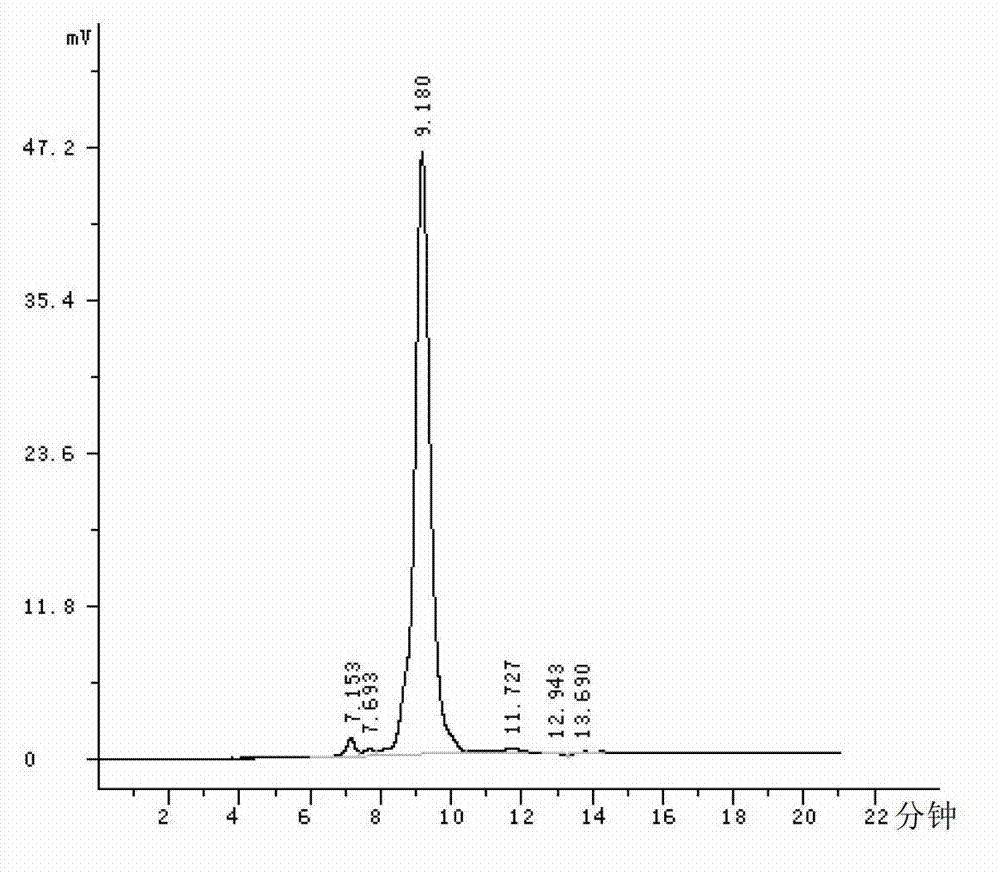
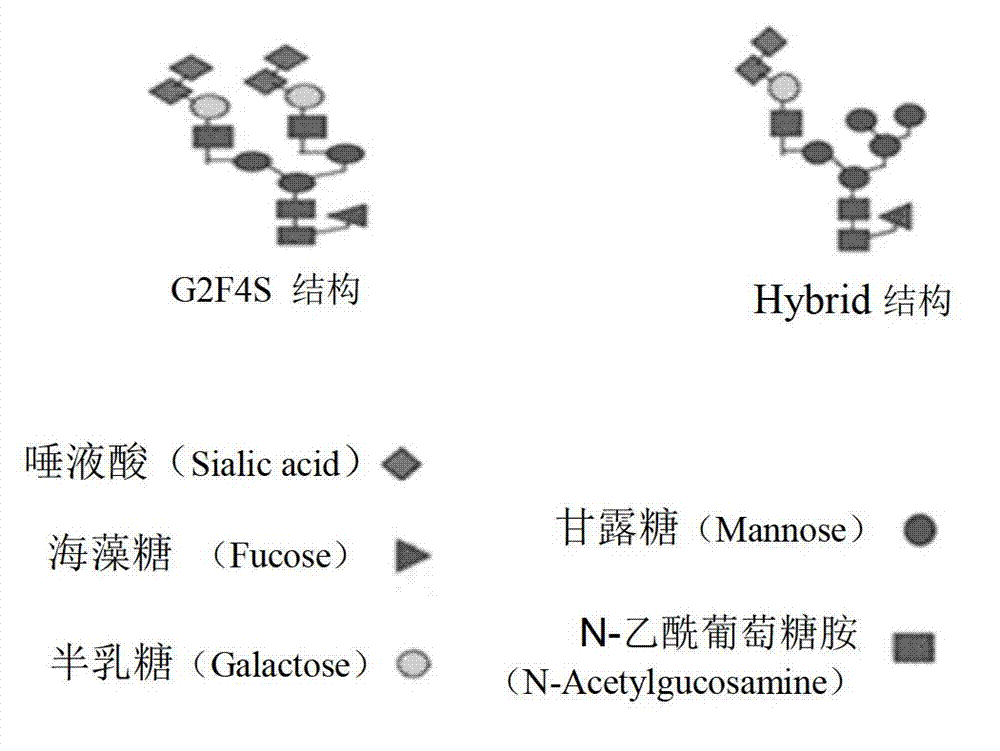
Agkistrodon acutus hemocoagulase-B
A technology of Agkistrodon akistrodon and hemagglutinase, applied in the field of serine protease, can solve the problem that blood clots cannot be dissolved by 5M urea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Purification of snake venom hemagglutinin-B
[0033] Take 30g of Agkistrodon acutus snake venom freeze-dried powder (batch number: 20090701, Guangxi Snake Venom Research Institute), and dissolve it in a chromatography cabinet at 4°C with a volume of 10 times the weight of the snake venom and pre-cooled 0.01M pH7.4 PBS for 30 minutes. Centrifuge at 4°C and 10000g for 15 minutes, pour the centrifugal supernatant into a dialysis bag, add 10 times the weight of snake venom to the pre-cooled PBS to stir and suspend the centrifugal sediment, and centrifuge again. Combine the two centrifugal supernatants in a dialysis bag (with a molecular weight cut-off of 10,000D), and dialyze 0.01M pH7.4 PBS in a chromatography cabinet at 4°C for 24 hours, changing the fluid 3 times during the period. Load the pre-treated snake venom solution onto a DEAE-Sepharose Fast Flow anion exchange chromatography column pre-equilibrated with 0.01M pH7.4PBS, wash the column with 0.01M pH7.4PBS,...
Embodiment 2
[0036] Example 2 Purification of Snake Venom Hemagglutinin-B
[0037] Take 30 g of Agkistrodon acutus snake venom freeze-dried powder (batch number: 20090701, Guangxi Snake Venom Research Institute), and perform pretreatment according to the same method as in Example 1. The pretreated snake venom solution was subjected to the first DEAE-Sephrose FF column chromatography according to the same method as in Example 1, and the target substance appeared in the elution peak of 0.06M NaCl by enzyme activity determination and electrophoresis analysis, and the elution collection solution was combined. A total of 2000ml, using Millipore Pellicon 2 tangential flow ultrafilter (0.1M 2 Cut off10k membrane) ultrafiltration and concentration to 210ml, pour the 210ml ultrafiltration concentrate into a dialysis bag (10,000D), dialyze with 2000ml 0.01M pH7.4PBS at 4°C for 24 hours, changing the solution 3 times during the period. The enzyme solution after dialysis was loaded onto the DEAE-Sephrose...
Embodiment 3
[0038] Example 3 Serine protein properties test of Agkistrodon acutus hemagglutinin-B
[0039] The hemagglutinin-B isolated in Example 1 was diluted with physiological saline to an enzyme activity of 1 U / ml.
[0040] Prepare 1% bovine fibrinogen (Sigma) solution with physiological saline.
[0041] Dissolve phenylmethylsulfonyl fluoride (PMSF, Merck) with isopropanol, the solution concentration is 4mg / ml.
[0042] The experimental operation steps are as follows:
[0043] (1) Take 2ml of 1% bovine fibrinogen solution and keep it at 37°C for 5 minutes.
[0044] (2) Take three small test tubes, labeled 1#, 2#, 3#, and add 200μl of 1U / ml hemagglutinin-C solution to each tube.
[0045] (3) Add 10μl of distilled water to 1# test tube, 10μl of isopropanol to 2# test tube, and 10μl of PMSF to 3# test tube, and keep them in a 37°C water bath for 5 minutes.
[0046] (4) Perform separate agglutination test observations in the order of test tube numbers. Add 200 μl of 1% bovine fibrinogen solution at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com