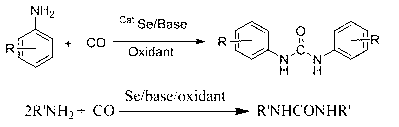
Method for synthesizing symmetrical urea compound
A technology for compounds and nitro compounds, applied in the preparation of organic compounds, chemical instruments and methods, preparation of urea derivatives, etc., can solve the problems of complex post-processing, high price, unfriendly environment, etc., and achieves low reaction process difficulty, The effect of simple operation and low equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add aniline (200mmol), nitrobenzene (40mmol), selenium powder (2mmol), and DBU (10mmol) into a 100ml three-neck flask equipped with a stirring bar and a condenser tube, continue to feed carbon monoxide, and heat to Stir and react at 95oC for 6 hours, after cooling to room temperature, switch carbon monoxide to air and stir for 0.5-1 hour, filter, wash with water, recrystallize with ethanol, and vacuum dry to obtain the target product diphenylurea.
[0025] The product has a purity of 100% and a yield of 40% as measured by proton nuclear magnetic resonance spectroscopy.
[0026]
[0027] white crystals; 1 H NMR ((CD 3 ) 2 SO; 300MHz) 8.656(2H,s,NH)7.436-7.469 (4H, d, Ar skeleton 1, 5, 6, 10 ), 7.252-7.305 (4H, t, ArH skeleton, 2, 4, 7, 9) , 6.941-6.994 (2H, t, ArH skeleton, 3,8) .
Embodiment 2
[0029] Add aniline (200mmol), selenium powder (2mmol), and DBU (10mmol) into a 100ml three-necked flask equipped with a stirring bar and a condenser tube, continuously feed carbon monoxide and oxygen, and heat to 95oC under normal pressure to react with stirring for 6 hours , after cooling to room temperature, switch carbon monoxide and oxygen to air and stir for 0.5-1 hour, filter, wash with water, recrystallize with ethanol, and dry in vacuum to obtain the target product diphenylurea.
[0030] The purity of the product measured by proton nuclear magnetic resonance is 100%, and the separation yield is 28%.
Embodiment 3
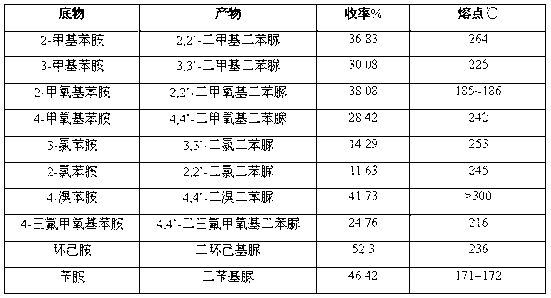
[0032] Only change reaction temperature, other conditions are with embodiment 1, and result is as table 1.
[0033] Table 1
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com