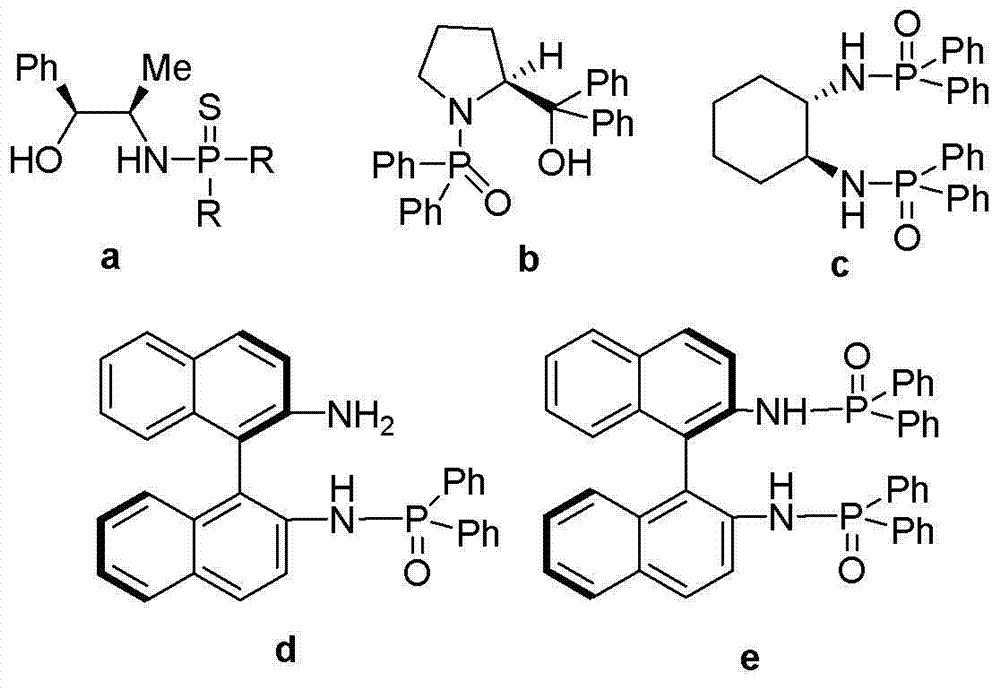
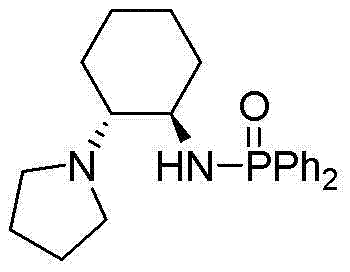
Cyclohexanediamine-derived phosphamide chiral ligand as well as preparation method thereof and application thereof
A technology of cyclohexanediamine and chiral ligands is applied in the field of new phosphoramide chiral ligands, which can solve the problems of harsh reaction conditions and large amount of chiral ligands.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of (1R,2R)-1-N,1-N-diethyl-2-N-(diphenylphosphoryl)-1,2-cyclohexanediamine:
[0030] In a 25 mL two-necked flask, add compound 4 (314 mg, 1.0 mmol), 5 mL acetonitrile, potassium carbonate (690 mg, 5.0 mmol), and iodoethane (780 mg, 5.0 mmol), and reflux for 4 h. TLC detection and tracking until the reaction is complete. Add 15 mL of saturated sodium bicarbonate solution, extract with dichloromethane (10 mL × 3), combine the organic phases, wash with saturated brine, dry with anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, and separate by flash column chromatography (wash The removal agent was methanol / dichloromethane (volume ratio 1:40) to obtain 293 mg of white solid, yield: 79%. Melting point: 112-113 o C; measured by optical rotation = -62.5 (c 1.00, CH 2 Cl 2 ); Nuclear magnetic analysis (Burker AVANCE 400 spectrometer): 1 H NMR (400 MHz, CDCl 3 ): δ 1.00 (t, J = 7.0 Hz, 6H), 1.06-1.26 (m, 4H), 1.45-1.53 (m, 1H),...
Embodiment 2
[0033] Example 2: Synthesis of (1R, 2R)-1-N-cyclopentyl-2-N-(diphenylphosphoryl)-1,2-cyclohexanediamine:
[0034] In a similar manner to Example 1, using 1,4-diiodobutane (620 mg, 2.0 mmol) instead of iodoethane (780 mg, 5.0 mmol), 313 mg of white solid was obtained, yield: 85%. Melting point: 99-101 o C; Rotation value measured = -31.8 (c 1.00, CH 2 Cl 2 ); Nuclear magnetic analysis (Burker AVANCE 400 spectrometer): 1 H NMR (400 MHz, CDCl 3 ): δ 0.91-1.41 (m, 5H), 1.44-1.60 (m, 1H), 1.63-1.88 (m, 6H), 1.92-2.06 (m, 1H), 2.44-2.84 (m, 4H), 3.11- 3.29 (m, 1H), 4.62-4.98 (br, 1H), 7.36-7.62 (m, 6H), 7.72-8.02 (m, 4H). 13 C NMR (100 MHz, CDCl 3 ): δ 21.8, 23.6, 24.1, 24.8, 33.8, 47.1, 53.0, 63.3 (d, J = 8.1 Hz), 128.1 (d, J = 12.7 Hz), 128.3 (d, J = 12.4 Hz), 131.2 (d , J = 9.7 Hz), 131.3 (d, J = 5.7 Hz), 131.4 (d, J = 11.1 Hz), 132.1(d, J = 9.4 Hz), 134.2 (d, J = 124.2 Hz), 134.4 (d , J = 130.2 Hz). 31 P NMR (162 MHz, CDCl 3 ): δ 22.7.
[0035]
[0036] The ligand is used to catal...
Embodiment 3
[0037] Example 3: Synthesis of (1R, 2R)-1-N-ethyl-2-N-(diphenylphosphoryl)-1,2-cyclohexanediamine:
[0038] In a method similar to Example 1, iodoethane (312 mg, 2.0 mmol) was used instead of iodoethane (780 mg, 5.0 mmol) to obtain 287 mg of white solid, yield: 85%. Melting point: 123-125 o C; Rotation value measured = -30.9 (c 1.00, CH 2 Cl 2 ); Nuclear magnetic analysis (Burker AVANCE 400 spectrometer): 1 H NMR (400 MHz, CDCl 3 ): δ 1.03-1.11 (m, 1H), 1.15-1.20 (m, 3H), 1.21-1.36 (m, 2H), 1.59-1.73 (m, 2H), 2.02-2.17 (m, 2H), 2.30- 2.49 (m, 2H), 2.49-2.62 (m, 2H), 2.74-2.97 (m, 2H), 3.51-3.65 (m, 1H), 7.41-7.60 (m, 6H), 7.80-8.00 (m, 4H) ); 13 C NMR (100 MHz, CDCl 3 ): δ 15.3, 24.6, 24.9, 31.1, 35.0 (d, J = 3.43 Hz), 40.9, 55.4, 62.8 (d, J = 5.29 Hz), 128.5 (q, J = 6.35 Hz), 131.7, 131.8, 132.1 , 132.2, 132.4, 132.6, 133.7, 133.8. 31 P NMR (162 MHz, CDCl 3 ): δ 26.6.
[0039]
[0040] The ligand is used to catalyze the asymmetric addition reaction of diethyl zinc to benzaldehy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com