Method for preparing polymer brush films with multiple stimulation responses
A multi-stimulus-responsive, polymer brush technology, applied in the homogeneous ATRP preparation of multi-stimulus-responsive polymer brush films, and the field of polymer brush film preparation, to achieve the effect of wide applicability and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 initiator 4-(2-bromoisobutyramide) butyltriethoxysilane
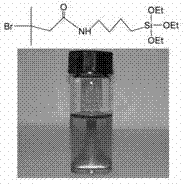
[0046] Add 12.6 ml of 4-aminobutyltriethoxysilane to 50 ml of anhydrous tetrahydrofuran, and transfer it to an ice-water bath at 0°C, add 10 ml of 2-bromoisobutyryl bromide dropwise, and then add 15 ml The acid-binding agent triethylamine was maintained in an ice-water bath for 2 hours, and then the reaction was transferred to room temperature for 24 hours. After filtration, the filtrate was rotary evaporated to completely remove THF to obtain a yellow-brown product. The calculated yield was 85%. Fig. 2 is its solution appearance and infrared spectrogram.
Embodiment 2
[0047] The synthesis of embodiment 2 initiator 3-(2-bromoisobutyramide) propyltriethoxysilane
[0048] Add 12.6 ml of 3-aminopropyltriethoxysilane to 50 ml of anhydrous tetrahydrofuran, and transfer it to an ice-water bath at 0°C, add 12.6 ml of 2-bromoisobutyryl bromide dropwise, and then add 14.3 ml Acid-binding agent triethylamine, after maintaining in an ice-water bath for 2 hours, transfer the reaction to room temperature and react for 24 hours. After filtration, the filtrate is rotary evaporated to remove tetrahydrofuran completely, and the yellow-brown product 3-(2-bromoisobutyramide) is obtained. Propyltriethoxysilane Initiator. Fig. 3 is its deuterated chloroform solution nuclear magnetic spectrum. NMR characterization: 1 H-NMR (CDCl 3 , 400MHz, TMS), δ: 6.89 (s, 1H, NH), 3.83 (q, 6H, J=6.8Hz, (CH 3 CH 2 O) 3 Si), 3.27 (q, 2H, J=6.8Hz, NCH 2 ), 1.95 (s, 6H, CH 3 C), 1.62~1.70 (m, 2H, CCH2), 1.23 (t, 9H, J=6.8Hz, (CH 3 CH 2 O) 3 Si), 0.65(t, 2H, J=8.4Hz, SiC...
Embodiment 3
[0050] The synthesis of embodiment 3 initiator 3-(2-bromoisobutyramide) propyltriethoxysilane
[0051] Except that the acid-binding agent was changed to pyridine, the same steps as in Example 2 were followed to obtain the tan product 3-(2-bromoisobutyramide)propyltriethoxysilane initiator. Fig. 3 is its deuterated chloroform solution nuclear magnetic spectrum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com