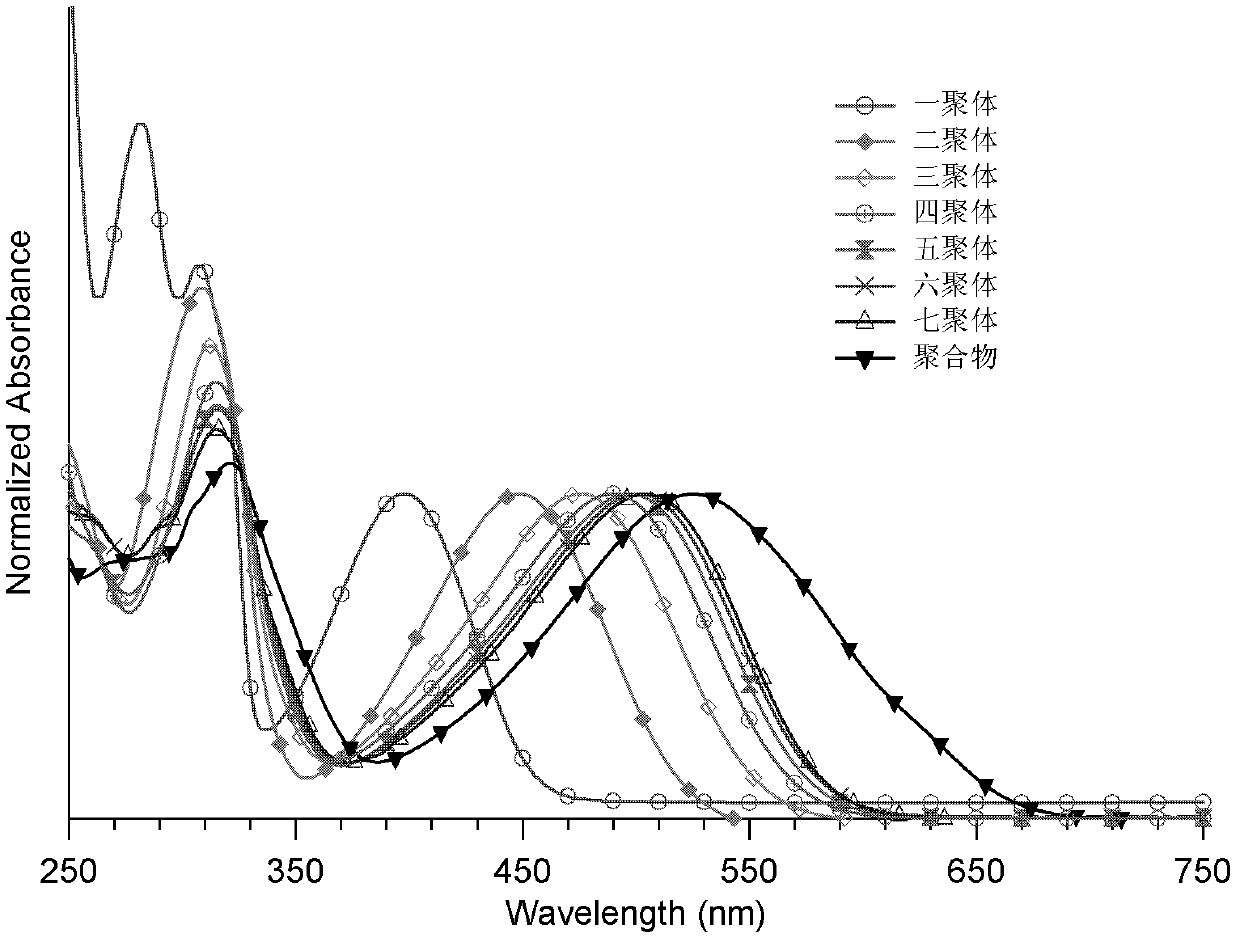
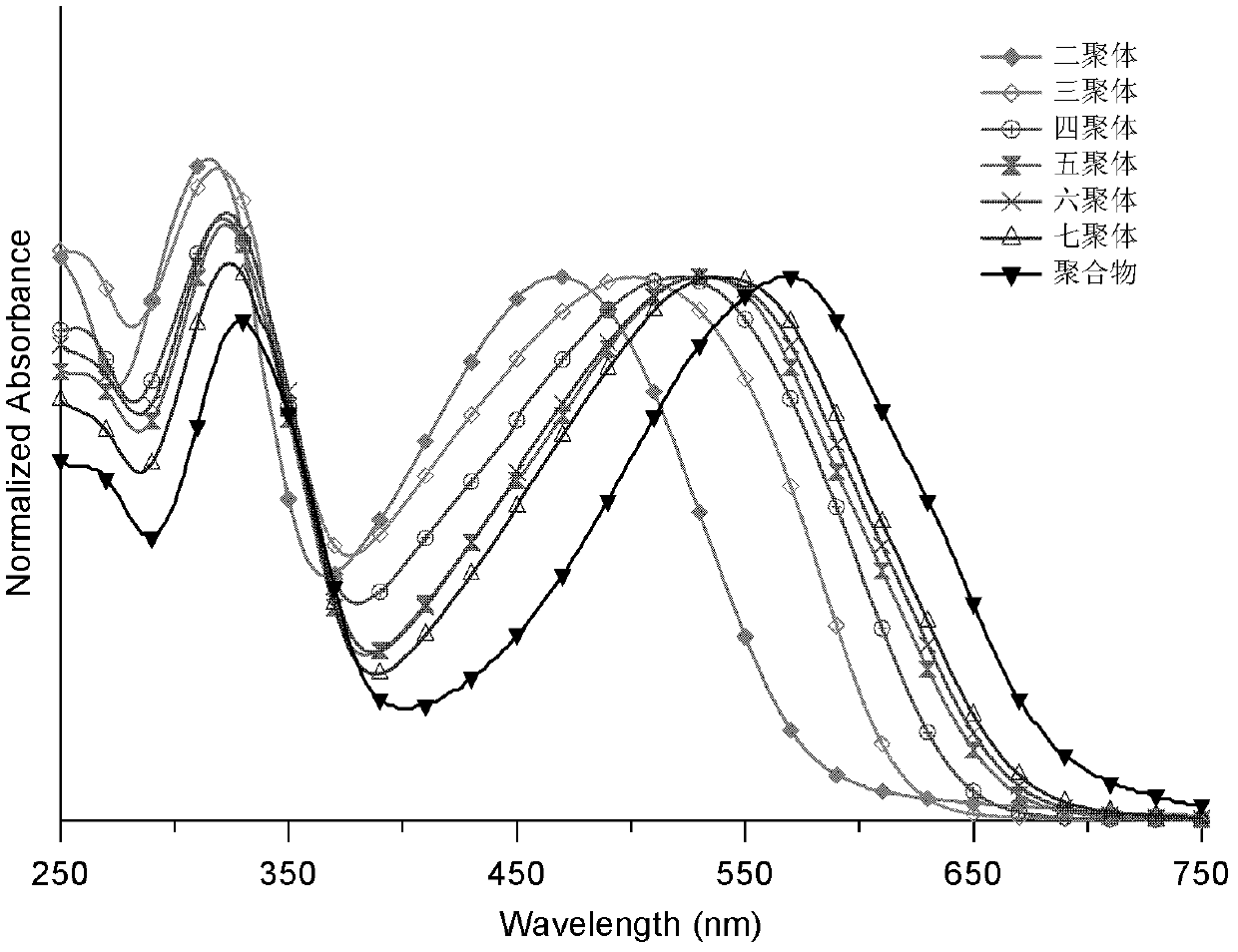
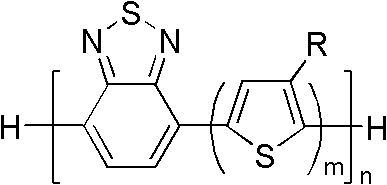
Conjugated polymer or oligomer having narrow band gap characteristic and side chain stereoregularity, and its preparation method
A technology of oligomers and polymers, which is applied in the field of organic conjugated polymers and oligomer materials and their preparation, can solve the problems of great differences, poor performance, and no consideration of side chain stereoregularity, etc., to achieve The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of monopolymer, namely 4-(4-alkyl-thiophen-2-yl)-[2,1,3]benzothiadiazole
[0033]
[0034] Step 1) In 40 mL of hydrobromic acid (HBr, 40%), add 4.29 g of [2,1,3]-benzothiadiazole. After heating to reflux, slowly add 1 equivalent (eq.) of liquid bromine (Br 2 , 2 mL), the reaction was continued for 3 hours under reflux conditions. After the temperature dropped to room temperature, sodium hydroxide (NaOH) aqueous solution was added to adjust the reaction mixture to neutrality, filtered, and the obtained solid was recrystallized by steam distillation and ethanol to obtain 1.94 g of 4-bromo-[2,1,3] - Benzothiadiazole, yield 29%. Step 2) Slowly drop 82.5 mL of n-butyllithium n-hexane solution (1.6 M) into a solution of diisopropylammonia (17.8 mL) in tetrahydrofuran (THF) under argon protection at -5°C, and stir for 0.5 h. Lithium diisopropylamide reagent (LDA) was prepared. Then, at -78°C, this reagent was slowly added dropwise into a THF solut...
Embodiment 2
[0036] Example 2: Preparation of the growth unit compound, namely 4-(4-alkyl-thiophen-2-yl)-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborin Pentane-2-yl)-[2,1,3]-benzothiadiazole
[0037]
[0038] Step 1) In 100 mL of hydrobromic acid (40%), add 14.9 g of [2,1,3]-benzothiadiazole. After heating to reflux, 3 equivalents of liquid bromine (16.8 mL) was slowly added dropwise, and the reaction was continued for 6 hours under reflux. After the temperature dropped to room temperature, NaOH aqueous solution was added, the reaction mixture was adjusted to neutrality, and the obtained yellow solid was recrystallized by THF to obtain 27.9 g of 4,7-dibromo-[2,1,3]-benzothiadiene azole with a yield of 82%.
[0039] Step 2) In 10 mL of THF, add 1 g (4-alkyl-thiophen-2-yl) sodium borate, 4.50 g 4,7-dibromo-[2,1,3]-benzothiadiazole, 2 mL Na 2 CO 3 aqueous solution (2M) and 0.14g Pd(PPh 3 ) 4 , After freezing and degassing, heat to 80°C for 12h. Then extracted with dichloromethane, separat...
Embodiment 3
[0041] Example 3: Preparation of dimers
[0042]
[0043] 0.62 g of N-bromosuccinimide (NBS) was gradually added to a THF solution of the monomer (1.06 g) under an ice-water bath. The reaction was carried out at room temperature for 12 hours, spin-dried and separated by column chromatography to obtain 118 g of brominated monomer with a yield of 88%.
[0044] In 10 mL of THF, add 1.02 g brominated monomer, 1.22 g 4-(4-alkyl-thiophen-2-yl)-7-(4,4,5,5-tetramethyl-1,3 , 2-dioxaborolan-2-yl)-[2,1,3]-benzothiadiazole, 4.1mLNa 2 CO 3 aqueous solution (2M) and 0.16g Pd(PPh 3 ) 4 , After freezing and degassing, heat to 80°C for 12h. Afterwards, it was extracted with dichloromethane and separated by column chromatography to obtain 1.24 g of dimer with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com