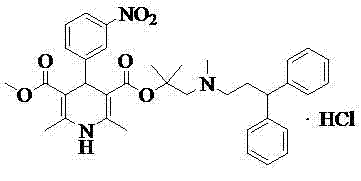
Refinement method of lercanidipine hydrochloride
A technology of lercanidipine hydrochloride and a purification method, applied in the field of medicine, can solve the problems of unstable yield and poor process reproducibility, and achieve the effects of short purification period, good stability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1, the preparation of lercanidipine hydrochloride crude product
[0017] Add 64g (0.193mol) lercanidipine hydrochloride core, 500mlDCM, 500mlDMF to the dry reaction flask, 2 Under protection, add 21ml (0.075mol) of thionyl chloride dropwise at 15°C~25°C, stir and react at 15°C~25°C for 2 hours, add dropwise dichloromethane solution of 57g (0.192mol) of lercanidipine hydrochloride side chain , stirred and reacted for 5 hours, after TLC showed that the reaction was complete, add 700ml saturated aqueous sodium bicarbonate solution to adjust PH=8, separate liquids, add 500ml of 4M dilute hydrochloric acid to the organic phase and stir to form a salt, evaporate to dryness under reduced pressure to obtain an oil, add 3000ml of isopropyl acetate was stirred for 10 hours for crystallization, and then filtered to obtain a light gray solid with a purity of 94.27%.
Embodiment 2
[0018] Embodiment 2, the volume determination contrast experiment of organic solvent
[0019] In a 250ml three-necked flask equipped with a thermometer and a stirring paddle, add 32 g of crude lercanidipine hydrochloride, and a mixed solvent of 48 ml of ethanol and 48 ml of isopropyl acetate, start stirring, raise the temperature to 30~35°C, soak and wash 2 hours, then lowered to room temperature (25°C), continued to stir for 6 hours, filtered, and air-dried at 60°C to obtain 28.2 g, yield 88.1%, HPLC purity 99.87%.
Embodiment 3
[0020] Embodiment 3, the volume determination contrast experiment of organic solvent
[0021] In a 250 ml three-necked flask equipped with a thermometer and a stirring paddle, respectively add 32 g of crude lercanidipine hydrochloride, add a mixed solvent of 24 ml of ethanol and 72 ml of isopropyl acetate, start stirring, heat up to 30~35 °C, soak Wash for 2 hours, then lower to room temperature (25°C), continue to stir for 6 hours, filter, and blow dry at 60°C to obtain 29.8 g, yield 93.1%, HPLC purity 99.91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com