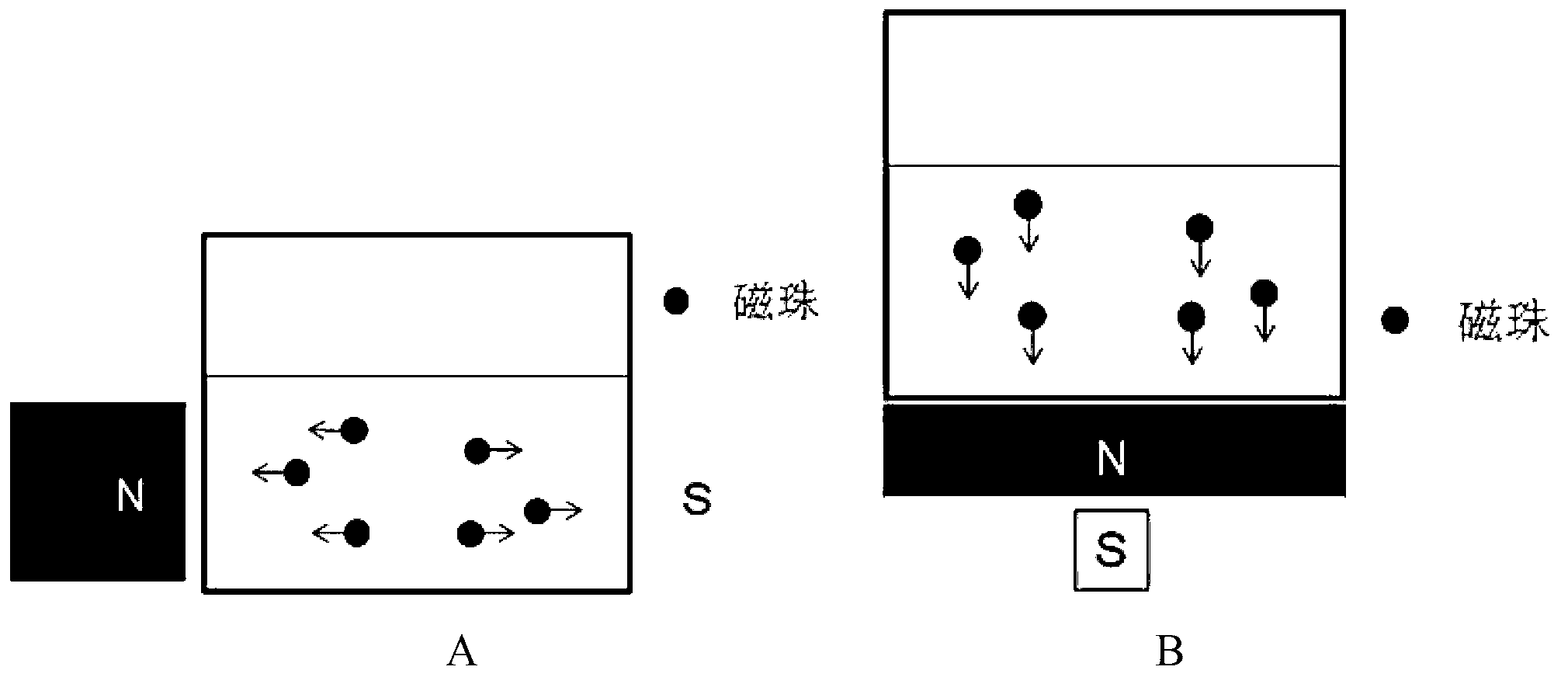
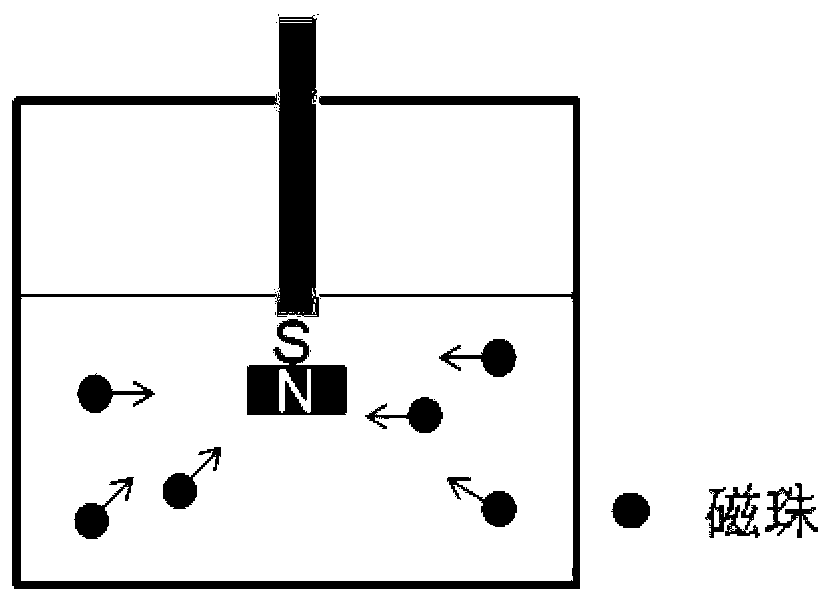
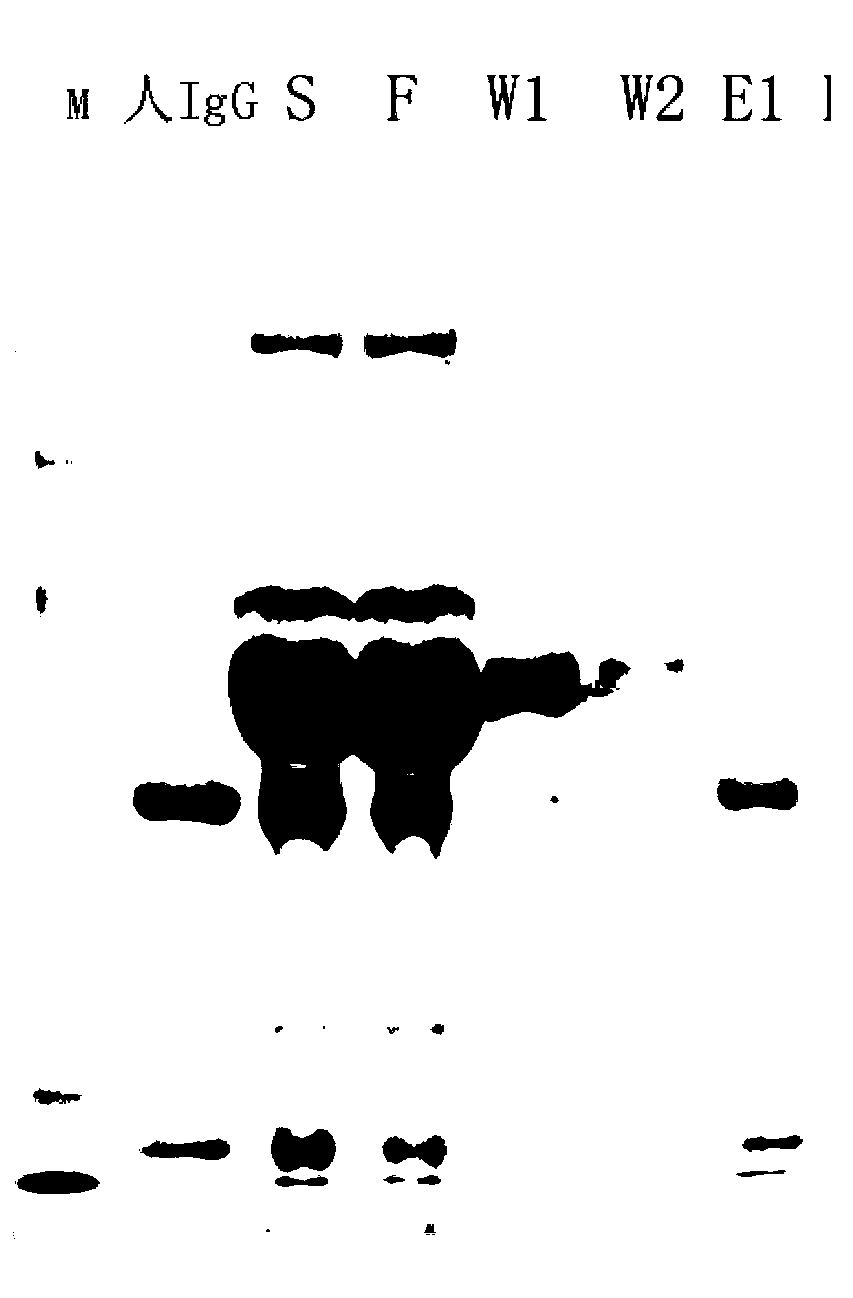Method for purifying antibodies using high-density peridium magnetic beads of protein A
A high-density, magnetic bead technology, applied in peptide preparation methods, chemical instruments and methods, immunoglobulins, etc., can solve the problems of long chromatographic operation, gap in antibody binding efficiency, and expensive protein A affinity purification packing. , to achieve the effect of saving production time and equipment requirements, improving antibody binding efficiency, and fast antibody capture speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Using SL-PA magnetic beads to extract human IgG from human serum, including the following steps:
[0044] For the preparation method of SL-PA magnetic beads, see Example 1 of the Chinese patent application "A method for directional immobilization of functional proteins using SLP" (patent application number: 201210107107.7).
[0045] (1) Take 100mg of SL-PA magnetic beads, add them to a 5ml centrifuge tube, then add 5ml of washing buffer (pH 7.5, containing 50mM phosphate buffer and 150mM NaCl; the same below), oscillate to resuspend, and place in a magnetic separator After 2 minutes, the supernatant was sucked off after the solid-liquid separation. Repeat this step once;
[0046] (2) Mix the washed magnetic beads with 3ml of human serum (purchased from Guangzhou Ruite Biotechnology Co., Ltd.) (that is, human serum S before purification), and incubate at room temperature for 10 minutes, shaking from time to time during the period to maintain the balance between the magn...
Embodiment 2
[0063] Extracting human IgG1 monoclonal antibody from CHO cell expression supernatant, including the following steps:
[0064] The recombinant CHO cell expression line containing human IgG1 gene (purchased from Guangzhou Yuansheng Pharmaceutical Technology Co., Ltd.) was taken, and after serum-free suspension culture, the recombinant CHO cell line expressed human IgG1 and secreted it into the culture supernatant, and the expression level was 1.15mg / ml. Take 20ml of the unfiltered culture solution and mix it with 100mg of SL-PA magnetic beads. The purification steps are the same as steps 1-7 of Example 1.
[0065] Results: 21.62 mg of human IgG1 was successfully extracted from the culture supernatant of recombinant CHO cells, the purification yield was 96%, the product purity was 94%, and the purification operation took 37 minutes.
[0066] The same volume and the same sample were purified using antibody affinity chromatography (chromatographic system: AKTA purifer 10, GE life...
Embodiment 3
[0069] Extract Rituximab monoclonal antibody from CHO cell mixture, including the following steps:
[0070] Take 20 mg of rituximab (purchased from Roche Pharmaceuticals, USA) and mix it with 20 ml of CHO cell culture medium to obtain a CHO cell mixture containing rituximab. Use 100 mg SL-PA magnetic beads to extract rituximab from this mixture, and the steps are the same as steps 1-7 in Example 1. As a result, 19.49 mg of rituximab was successfully extracted from the mixture, with a purification yield of 97.45% and a purity of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com