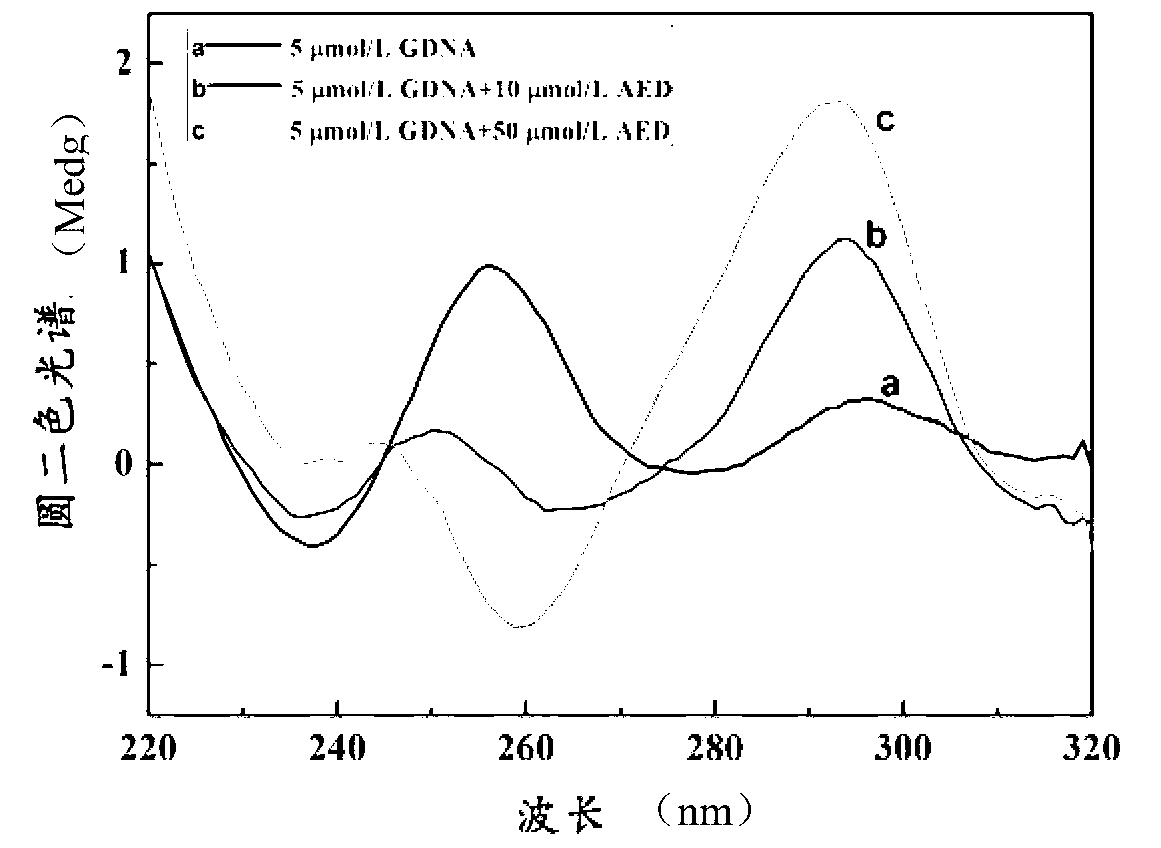
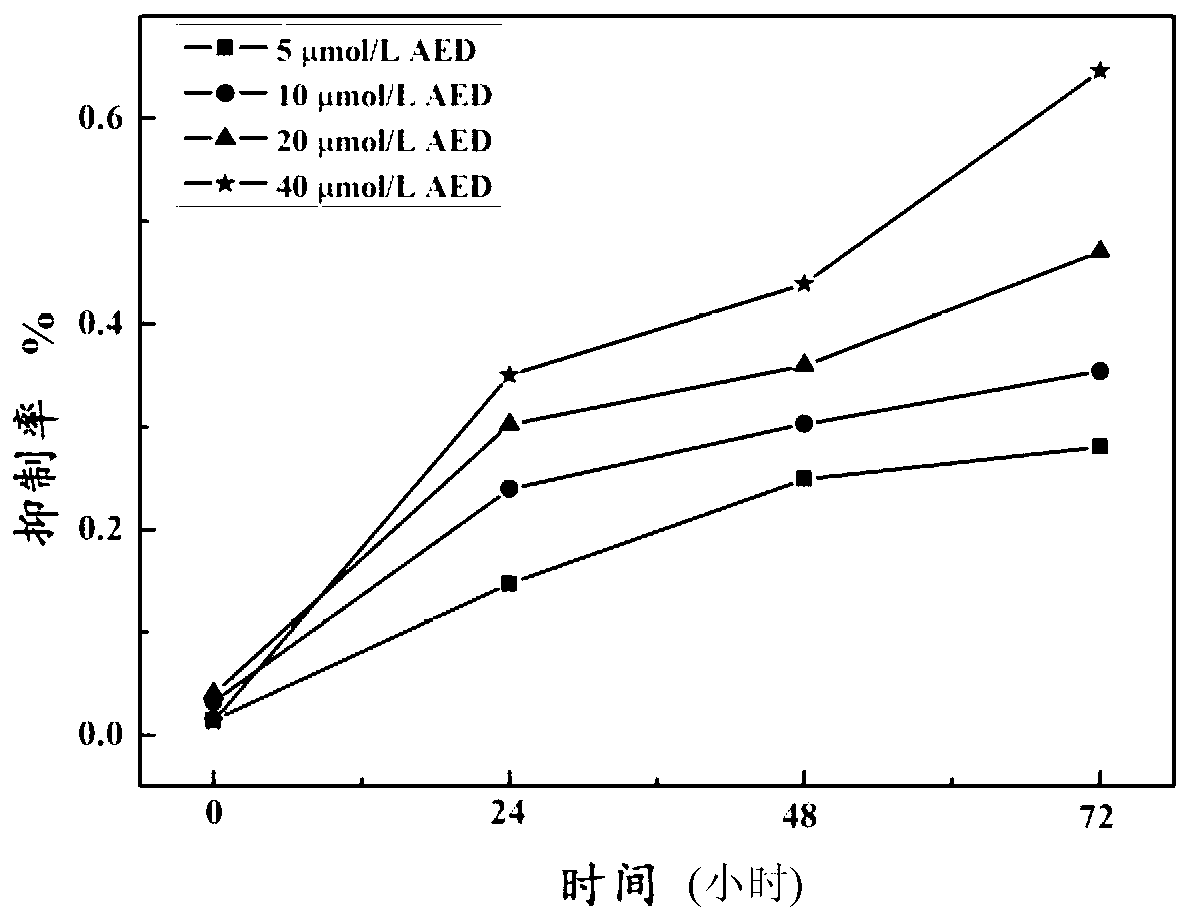
Aloe emodin derivative and preparation method thereof
A technology of aloe-emodin and derivatives, which is applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc., can solve the problems of gastrointestinal stimulation, difficulty of aloe-emodin, poor bioavailability, etc., and achieve less by-products and easy preparation Simple method, water-soluble and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
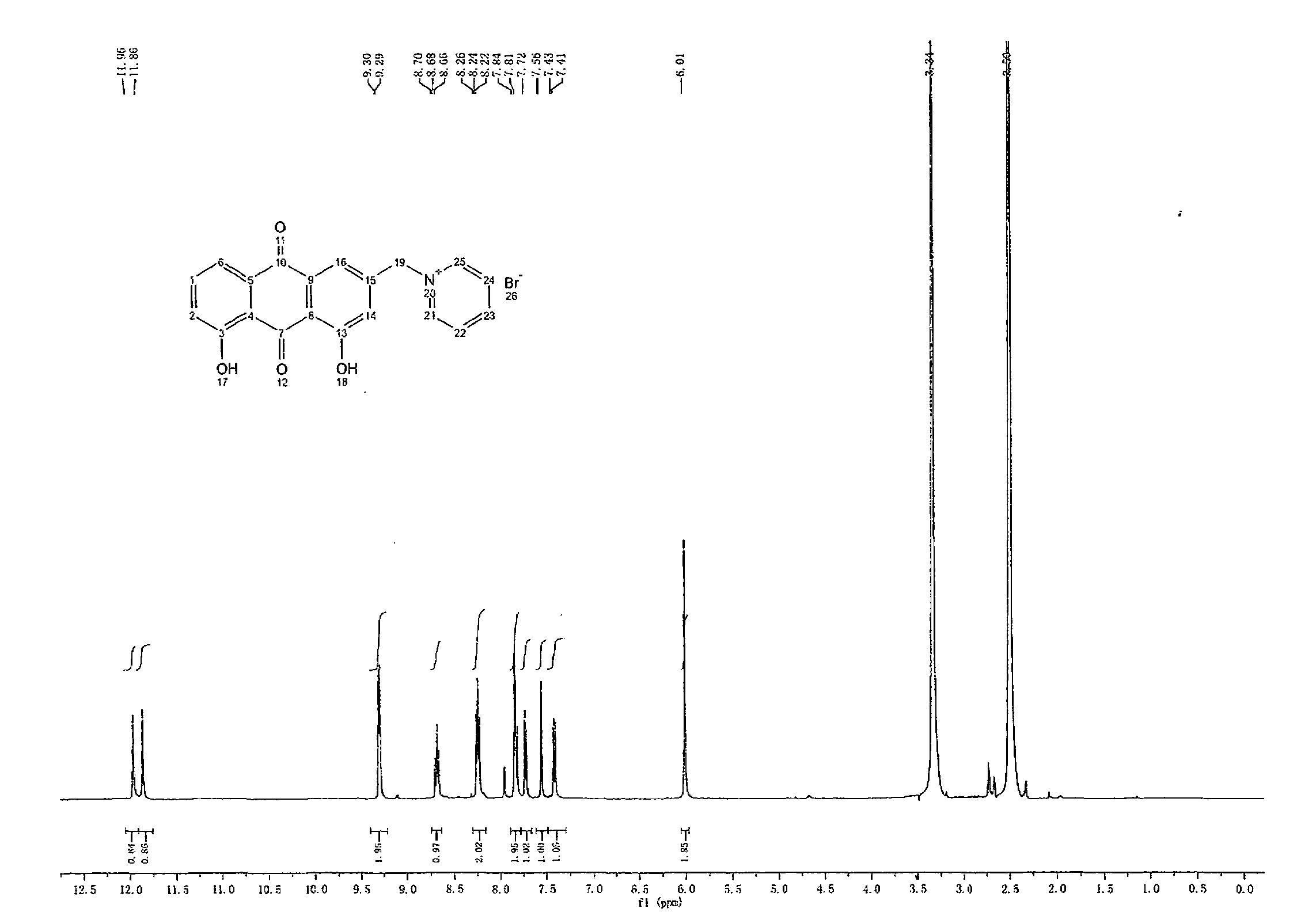
[0029] Take the synthesis of 1-[(9,10-dihydro-1,8-dihydroxy-9,10-dioxo-3-anthracenyl)methyl]pyridinium bromide as an example, molecular mass: 411.0, structural formula for:
[0030] Formula 1)
[0031] where R is
[0032] The preparation method of above-mentioned 1-[(9,10-dihydro-1,8-dihydroxyl-9,10-dioxo-3-anthracenyl)methyl]pyridinium salt bromide comprises the following steps:
[0033] (1) Synthesis of 3-bromomethyl-1,8-dihydroxy-9,10-anthraquinone
[0034] Add 0.50g of aloe-emodin to 18.5mL of dry dichloromethane, add 1.93g of triphenylphosphine and 2.45g of carbon tetrabromide to the reaction solution at 0°C, The molar ratio of carbonized carbon was 1:4:4, stirred at room temperature for 2.5 hours, filtered, the filtrate was concentrated, and the residue was purified by silica gel column chromatography to obtain 0.52g of 3-bromomethyl-1,8-dihydroxy-9, 10-anthraquinone, the chemical reaction formula is:
[0035] Formula 1)
[0036] (2) Synthesis of aloe-emodin ...
Embodiment 2
[0042] Taking the synthesis of 1-[(9,10-dihydro-1,8-dihydroxy-9,10-dioxo-3-anthracenyl)methyl]pyridinium bromide as an example, the preparation method comprises the following steps:
[0043] (1) Synthesis of 3-bromomethyl-1,8-dihydroxy-9,10-anthraquinone
[0044] Add 0.5g of aloe-emodin to 18.5mL of dry dichloromethane, add 1.45g of triphenylphosphine and 1.84g of carbon tetrabromide to the reaction solution at 0°C, The molar ratio of carbonized carbon was 1:3:3, stirred at room temperature for 2.5 hours, filtered, the filtrate was concentrated, and the residue was purified by silica gel column chromatography. The chemical reaction formula was formula (1), and 0.50 g of 3-bromomethyl- 1,8-Dihydroxy-9,10-anthraquinone.
[0045] (2) Synthesis of aloe-emodin derivatives
[0046] Dissolve 0.2 g of 1,8-dihydroxy-3-bromomethyl-9,10-anthraquinone prepared in step (1) in 6 mL of dry N,N-dimethylformamide, add 0.15 mL of pyridine , the molar ratio of 3-bromomethyl-1,8-dihydroxy-9,10...
Embodiment 3
[0048] Taking the synthesis of 1-[(9,10-dihydro-1,8-dihydroxy-9,10-dioxo-3-anthracenyl)methyl]pyridinium bromide as an example, the preparation method comprises the following steps:
[0049] (1) Synthesis of 3-bromomethyl-1,8-dihydroxy-9,10-anthraquinone
[0050] Add 0.5g of aloe-emodin to dry dichloromethane to dissolve completely, add 2.42g of triphenylphosphine and 3.06g of carbon tetrabromide to the reaction solution in turn at 0°C, aloe-emodin and triphenylphosphine, tetrabromide The molar ratio of carbonized carbon is 1:5:5, stirred at room temperature for 2.5 hours, filtered, the filtrate is concentrated, and the residue is purified by silica gel column chromatography. The chemical reaction formula is formula (1), and 0.49 g of 3-bromomethyl- 1,8-Dihydroxy-9,10-anthraquinone.
[0051] (2) Synthesis of aloe-emodin derivatives
[0052] Dissolve 0.2 g of 3-bromomethyl-1,8-dihydroxy-9,10-anthraquinone obtained in step (1) in 7 mL of dry N,N-dimethylformamide, add 0.24 mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com