Preparation method of iron-containing lithium-rich manganese-based positive electrode material
A cathode material, lithium-rich manganese-based technology, applied in the field of lithium ion cathode materials and electrochemistry, can solve the problems of high equipment requirements, high operating temperature, hindering the promotion of iron-containing lithium-rich manganese-based cathode materials, etc., to meet the needs of large-scale requirements, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
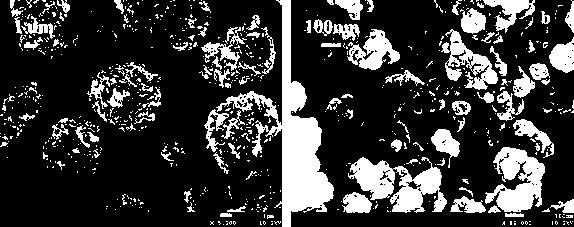
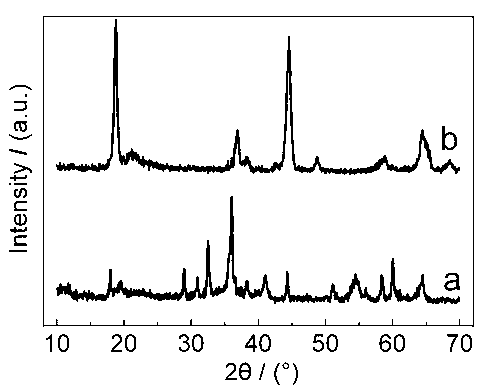
Image
Examples
Embodiment 1
[0025] FeSO 4 ·7H 2 O and MnSO 4 ·H 2 O is dissolved in deionized water at a molar percentage of 1:3, and the concentration of the mixed solution is 1.0 mol / L; prepare a precipitant with the same volume as the sulfate mixed solution, with a concentration of 2 mol / L NaOH solution, and add a small amount of Ammonia water, the concentration of ammonia water is 0.1 mol / L. Add 800 ml of deionized water to a 2 L beaker, maintain the temperature at 50 °C, and use an electric stirrer to continuously stir the deionized water, then pump the sulfate mixed solution and precipitant into the deionized water at a flow rate of 10 ml / min at the same time , for co-precipitation reaction. The resulting coprecipitate was washed, filtered and dried to obtain the precursor. According to the molar ratio of lithium to iron and manganese molar ratio of 1.7:1, the lithium hydroxide is mixed with the precursor evenly. Finally, the mixture of lithium hydroxide and the precursor was kept in a tubula...
Embodiment 2
[0027] FeSO 4 ·7H 2 O and MnSO 4 ·H 2 O is dissolved in deionized water at a molar percentage of 1:3, and the concentration of the mixed solution is 1.0 mol / L; the precipitant with the same volume as the sulfate mixed solution is prepared, and the concentration is 1 mol / L of Na 2 CO 3 solution. Add 800 ml of deionized water to a 2 L beaker, maintain the temperature at 50 °C, and use an electric stirrer to continuously stir the deionized water, then pump the sulfate mixed solution and precipitant into the deionized water at a flow rate of 10 ml / min at the same time , for co-precipitation reaction. The resulting coprecipitate was washed, filtered and dried to obtain the precursor. According to the molar ratio of lithium to iron and manganese molar ratio of 1.7:1, the lithium hydroxide is mixed with the precursor evenly. The mixture of lithium hydroxide and precursor is kept in a tubular calciner at 500 °C for 5 hours, then the temperature is raised to 600 °C, kept at 12 h...
Embodiment 3
[0029] FeSO 4 ·7H 2 O and MnSO 4 ·H 2 O and NiSO 4 ·6H 2 The total number of moles of O is dissolved in deionized water at a molar percentage of 1:3, and the concentration of the mixed solution is controlled at 1.0 mol / L; a precipitant with the same volume as the sulfate mixed solution is prepared, and the concentration is 2 mol / L of NaOH solution. A small amount of ammonia water was added to the solution, and the concentration of ammonia water was 0.1 mol / L. Add 800 ml of deionized water into a 2 L beaker, keep the temperature at 60 °C, and use an electric stirrer to continuously stir the deionized water, then pump the sulfate mixed solution and precipitant into the deionized water at a flow rate of 10 ml / min at the same time , for co-precipitation reaction. The resulting coprecipitate was washed, filtered and dried to obtain the precursor. According to the ratio of moles of lithium to moles of iron and manganese of 1.55:1, lithium hydroxide is mixed with the precursor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com