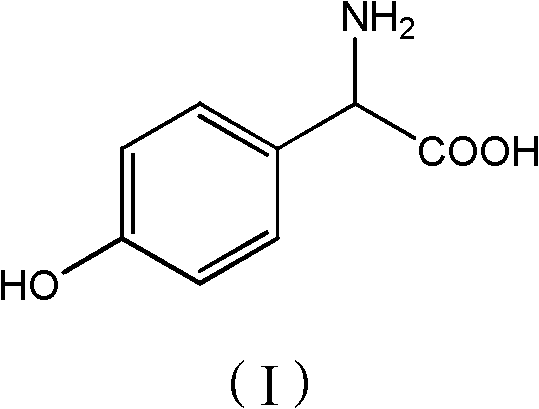
P-hydroxy phenylglycine synthesis method
A technology of p-hydroxyphenylglycine and hydroxyglycine, applied in the field of medicine and chemical industry, can solve the problems of many operation steps, high environmental protection pressure, few steps, etc., and achieve the effects of simple operation process, high equipment utilization rate and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
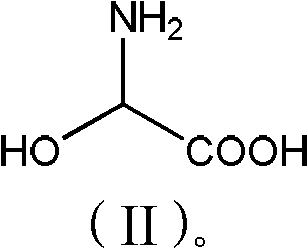
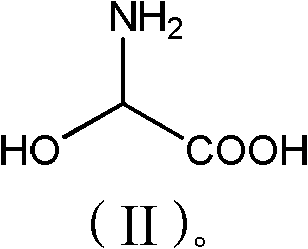
[0028] Add phenol (56.4g, 0.6mol) and 2-hydroxyglycine (45.5g, 0.50mol) to 150g methanol, stir well, add p-toluenesulfonic acid (10g, 0.06mol), heat up to 55-60°C for reaction, TLC Detection, after 6 hours of reaction, methanol was concentrated and recovered, 100g of water was added to the concentrated solution, neutralized to pH 3.5 with ammonia water, cooled to 20°C, filtered, washed with 200g of water, and dried to obtain 61.3g of product with a purity of 98%. 72%.
[0029] p-Hydroxyphenylglycine 1 H-NMR data: 1 H-NMR (400MHz, D 2 O) δ: 7.18 (d, J=8Hz, 2H), 6.80 (d, J=8Hz, 2H), 4.59 (s, 1H).
Embodiment 2
[0031] Add phenol (56.4g, 0.6mol) and 2-hydroxyglycine (45.5g, 0.50mol) to 150g toluene, stir well, add acetic acid (6g, 0.1mol), heat up to 75-80 ℃ reaction, TLC detection, 10 After the reaction in 1 hour, add 200g of water, separate the organic phase, neutralize the aqueous phase with sodium carbonate to pH 6.8, cool to 30°C, filter, wash with 150g of water, and dry to obtain 64.2g of product with a purity of 97.5% and a yield of 75%. .
Embodiment 3
[0033] Add phenol (56.4g, 0.6mol) and 2-hydroxyglycine (45.5g, 0.50mol) to 150g dichloroethane, stir evenly, add sulfuric acid (10g, 0.10mol), heat up to 60-65°C for reaction, TLC detection After 8 hours of reaction, add 150g of water, separate the organic phase, neutralize the water phase with aqueous sodium hydroxide to pH 5.2, cool to 15°C and filter, wash with 250g of water, and dry to obtain 68.2g of product with a purity of 98%. Yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com