Serotype 5 swine actinobacillus pleuropneumoniae (APP) Apx I C/Apx II C double gene deleted vaccine candidate strain
A porcine pleuropneumonia and missing vaccine technology, applied in the direction of bacteria, microbe-based methods, microbiological measurement/inspection, etc., can solve the problems of vaccines that are only suitable for basic research, do not meet biosafety requirements, and have no cross-protection, and reach a broad range Market application prospect, highly targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
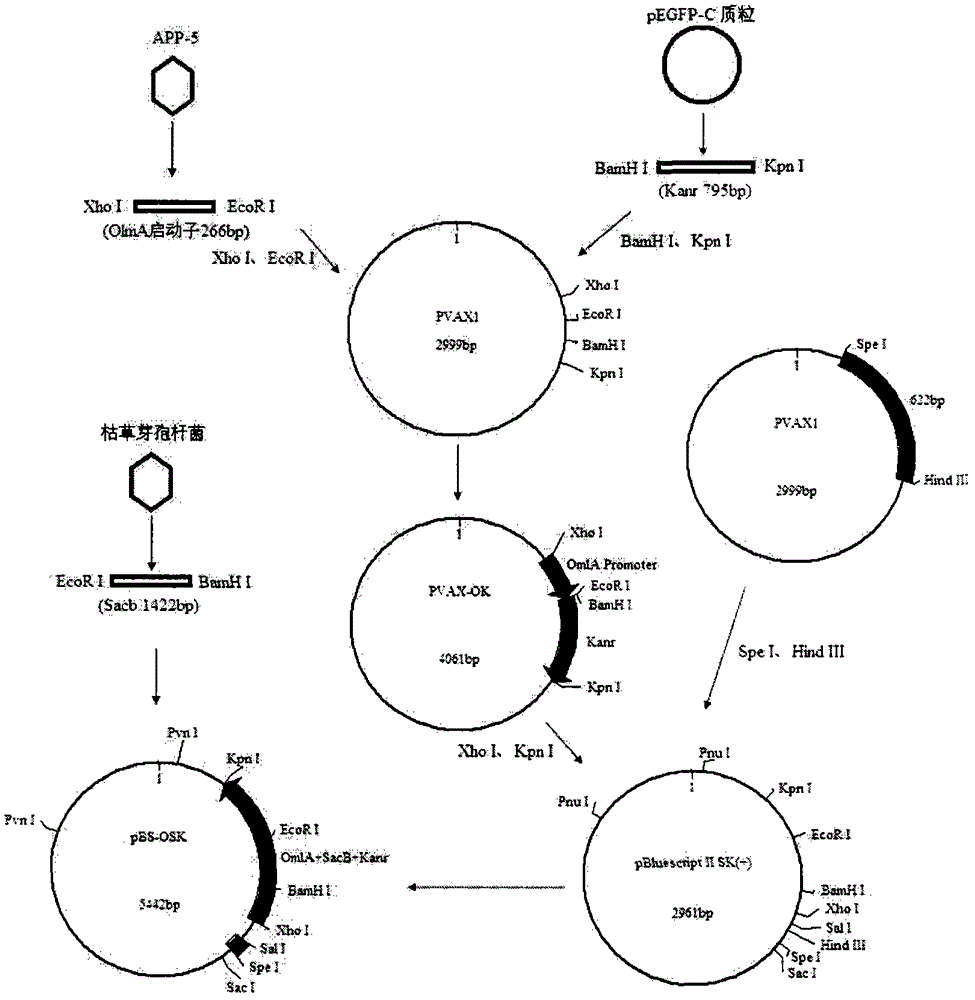
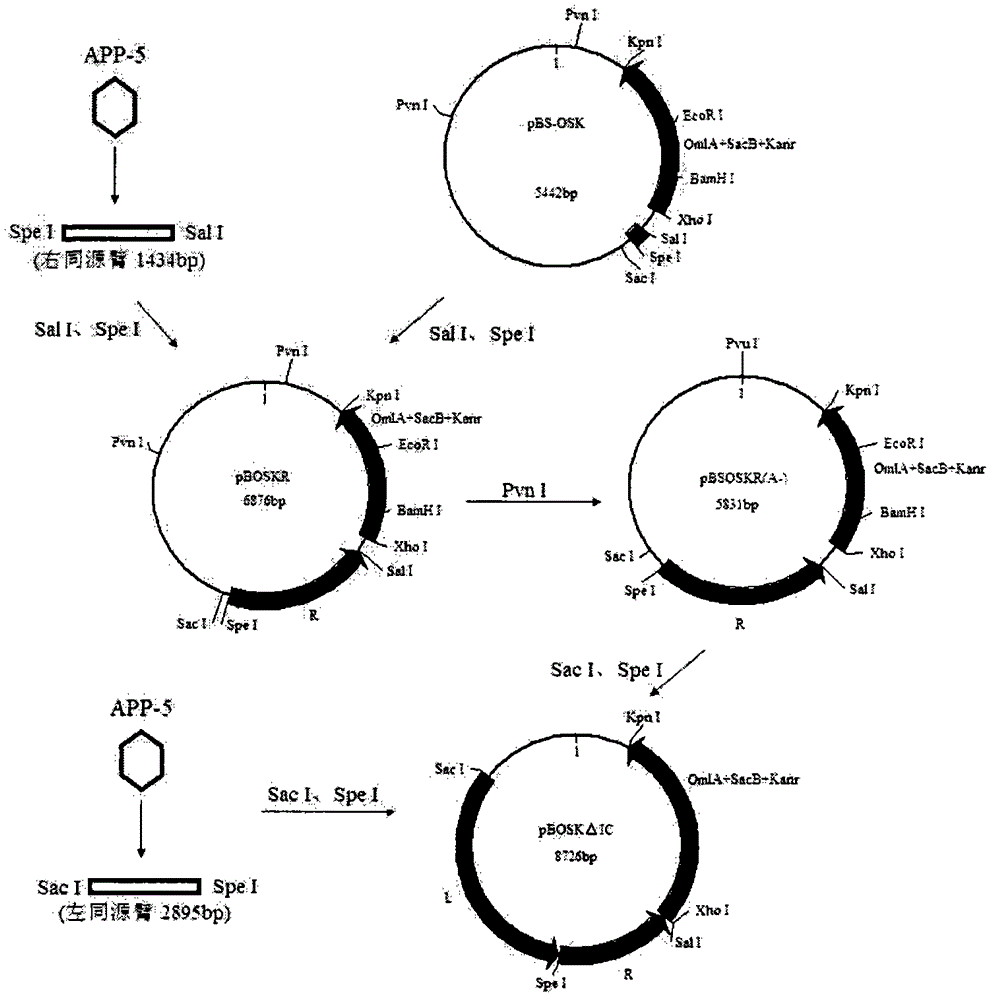
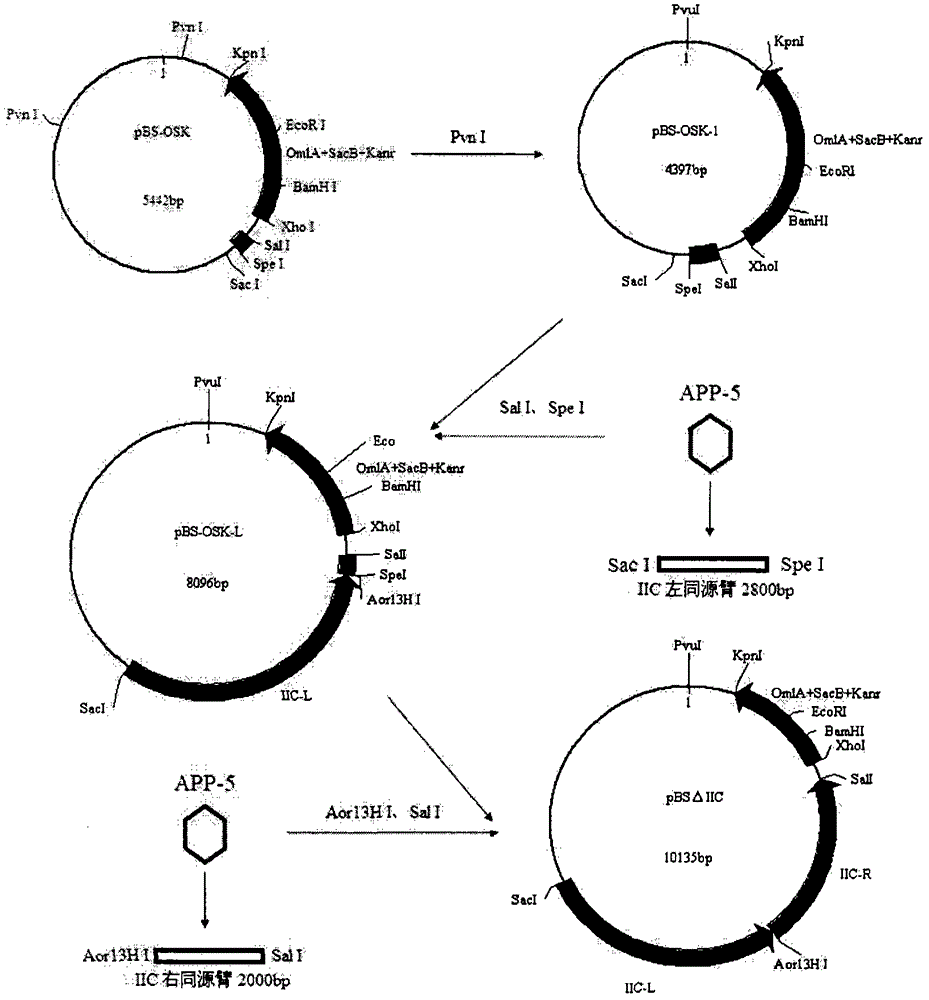
[0029] Example 1. Construction of double gene deletion strain SW1ΔI C / ΔII C ( image 3 )
[0030] SW1 strain, Escherichia coli DH5α, BL21, Bacillus subtilis PKC01 strain, and Actinobacillus pleuropneumoniae serotype 5 strain K17 were purchased from China Veterinary Drug Control Institute.
[0031] Escherichia coli was cultured in LB liquid or solid medium, and ampicillin (Amp) or kanamycin (Kan) with a final concentration of 100 μg / ml was added according to different needs; infectious pleuropneumonia actinic rods were cultured in TSB liquid Cultured in medium or TSA solid medium, and added NAD at a final concentration of 10 μg / ml.
[0032] pBluescrIpt II SK+, pVAX1, PCR product cloning vector pMD19-TSimple were purchased from Treasure Bioengineering (Dalian) Co., Ltd.
[0033] Plasmid mini-extraction kit and DNA gel recovery kit were purchased from OMEGA Company. Taq DNA polymerase and DNA Marker were purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd. Variou...
Embodiment 2
[0110] Example 2. Research on the biological characteristics of the double-gene deletion strain SW1ΔI C / ΔII C
[0111] (1) Growth characteristics test
[0112] Inoculate the single colonies of the double-gene deletion strain (SW1ΔI C / ΔII C) and the parental strain (SW1) in TSB liquid medium for overnight culture, then inoculate 50 μL of the above bacterial liquid into 50 mL of TSB liquid medium for culture, and wait until the OD 600 When the value is 0.13, start to measure, take a sample every 1h, and read its OD with a nucleic acid protein analyzer 600 value, through each time point OD 600 value to compare their growth rates. According to OD 600 The growth curves of the parental strain and the gene deletion strain were drawn to compare the growth characteristics of the two.
[0113] The growth curves of double-gene deletion strains and parent strains are as follows: Figure 8 shown. It can be seen from the growth curve that the growth of the gene deletion strain and the...
Embodiment 3
[0120] Example 3. Preparation of double gene deletion strain (SW1ΔI C / ΔII C) attenuated vaccine
[0121] Inoculate the gene-deficient strain into a test tube containing 5mL TSB culture medium and recover for 6-8h, then streak inoculate it on a TSA plate and culture it at a constant temperature at 37°C overnight, pick a well-growing single colony and inoculate it into 100mL TSB culture on the next day Expand the culture on the base, when the OD of the bacterial solution 600 When it reaches about 2.0, the number of viable bacteria is about 6×10 9 CFU / mL, at this time, mix the bacterial solution and 20% skim milk (sterilized) in the ultra-clean workbench at a ratio of 1:1, pack in 2 mL / bottle, seal with sterilized absorbent cotton, and place in- After freezing in a refrigerator at 20°C for 24 hours, freeze and vacuum-dry them in a freeze dryer at -50°C, then take them out, cover them, and store them at -20°C. At the same time, the finished product inspection of the vaccine is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com