Double trifluoromethyl substituent-containing asymmetric aromatic diamine monomer and preparation method thereof
A bistrifluoromethyl and trimethylhydroquinone technology, which is applied in the field of fluorine-containing aromatic diamine compounds and its preparation, can solve the problems of polyimide dark color and poor optical transparency, and achieve easy purification and separation , Excellent optical properties, excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
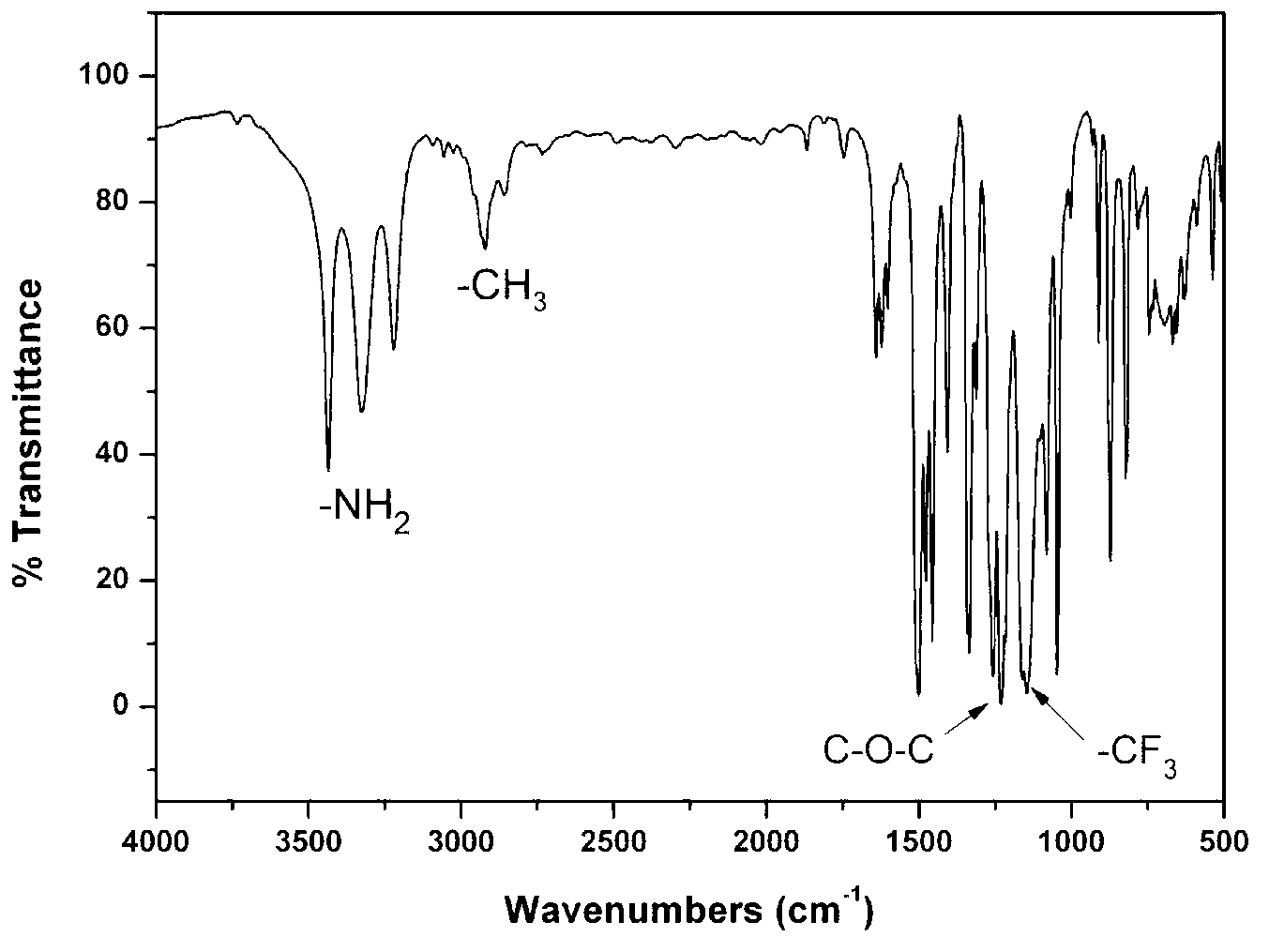
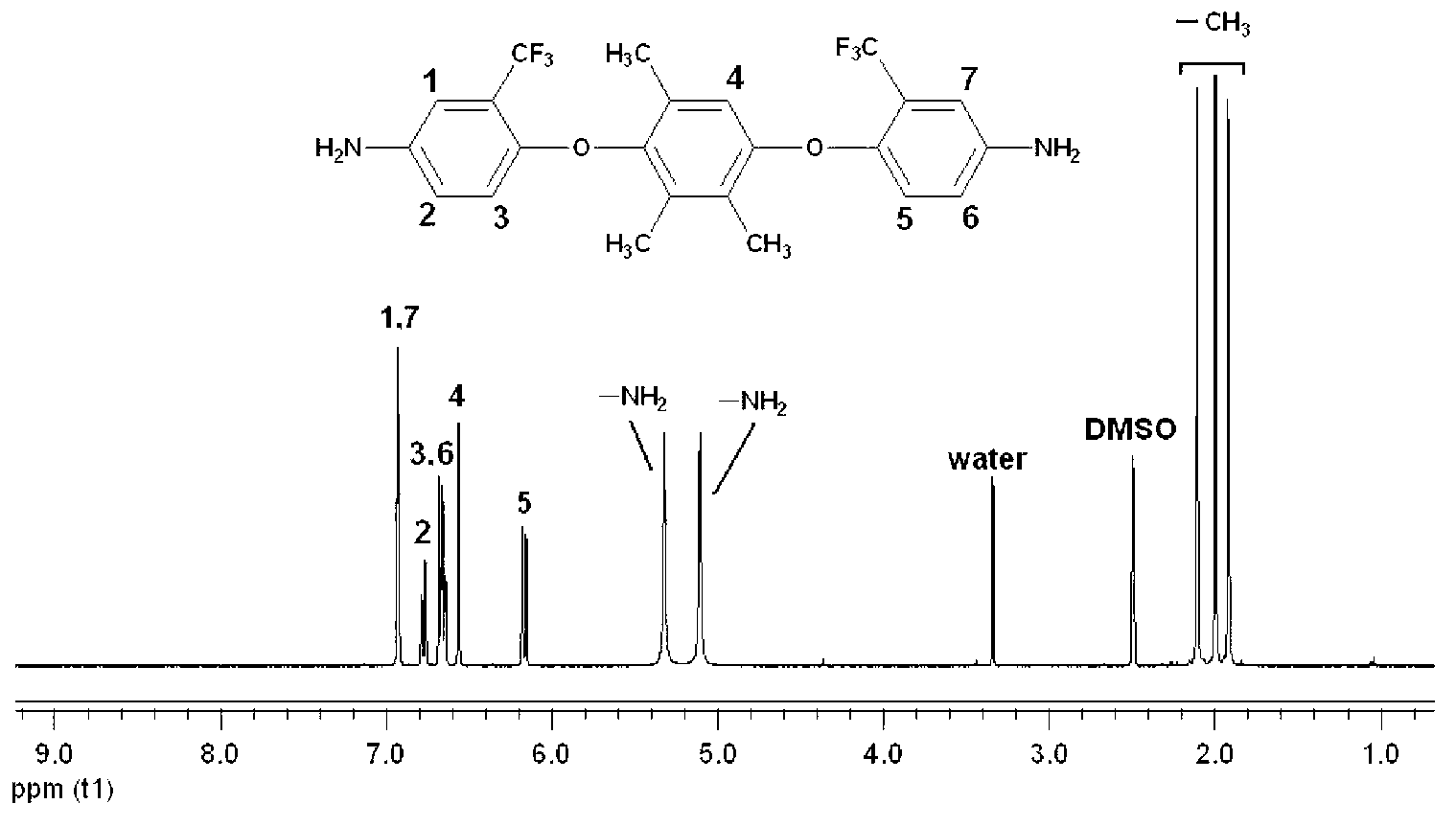
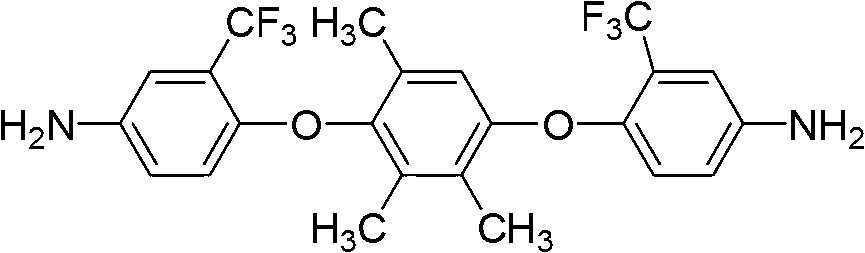
[0024] Preparation of 1,4-bis(4-amino-2-trifluoromethylphenoxy)-2,3,5-trimethylbenzene:
[0025] ①Under nitrogen protection, add 15.22g (0.1mol) 2,3,5-trimethylhydroquinone and 45.11g (0.2mol) 2-chloro-5-nitrobenzotrifluoride into a 500ml three-necked flask Add 28.98g (0.21mol) of potassium carbonate and 130ml of N,N-dimethylformamide respectively, react at 120°C for 12h, pour the product into 1000ml of methanol / water (1:1) and fully stir, precipitate and filter. The product was washed repeatedly with hot water, and further recrystallized with NN-dimethylformamide / methanol (3:2) mixed solvent to obtain a light yellow fluorine-containing dinitro compound: 1,4-bis(4-nitro-2- Trifluoromethylphenoxy)-2,3,5-trimethylbenzene, the yield is 85%, and the melting point is 256-257°C. 1 H NMR (DMSO-d 6 ,400MHz)δ:8.64(d,J=2.8Hz,1H),8.62(d,J=2.8Hz,1H),8.32(dd,J 1 =2.8Hz,J 2 =9.2Hz,1H),8.30(dd,J 1 =2.8Hz,J 2 =9.2Hz,1H),6.91(s,1H),6.80(d,J=9.2Hz,1H),6.62(d,J=9.2Hz,1H),2.13(s,3H),2.11(s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com