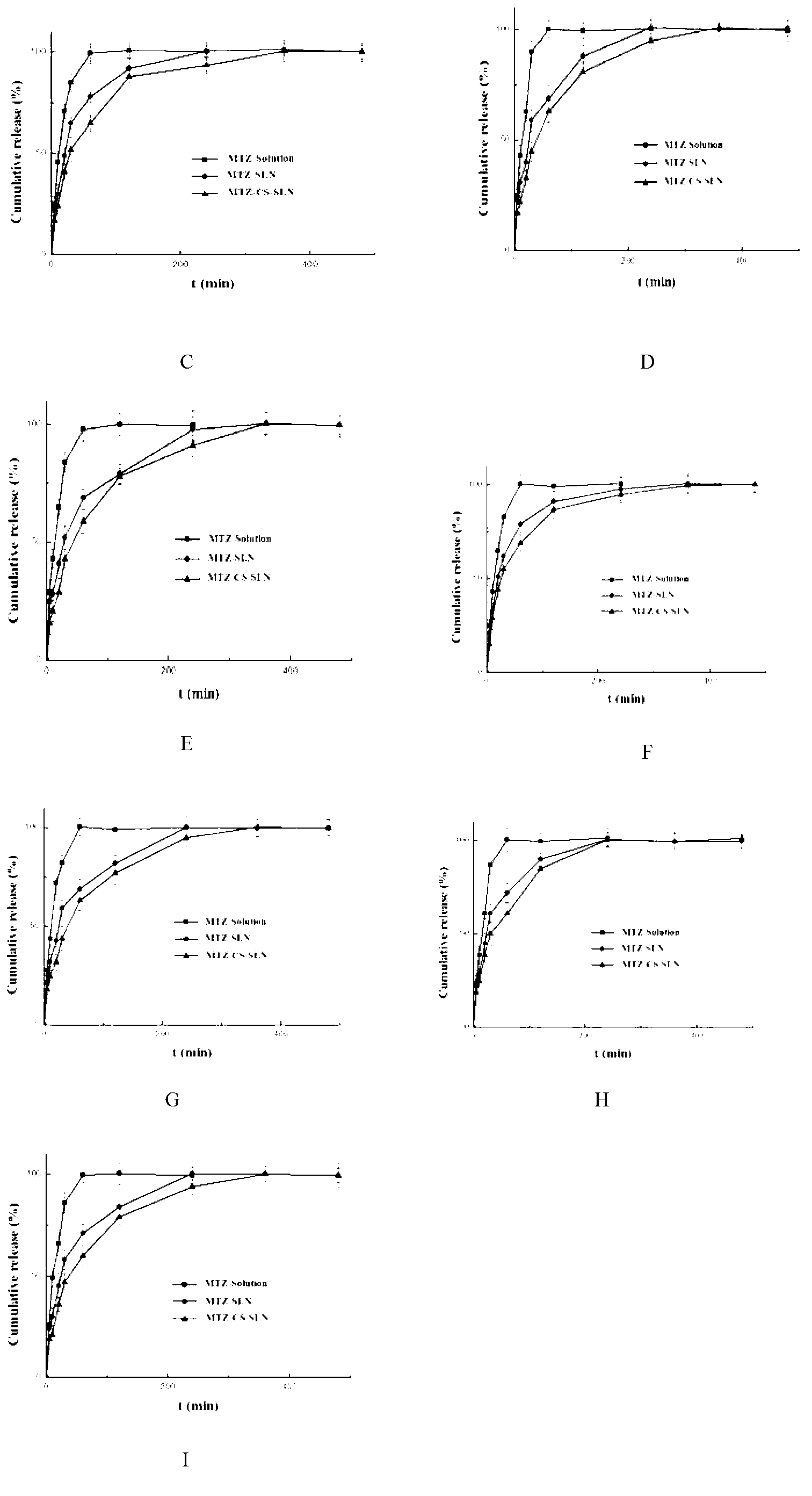Chitosan-modified methazolamide solid lipid nanoparticles and preparation method thereof
A technology of solid lipid nanometer and methazolamide, which is applied in sensory diseases, powder delivery, drug combination, etc., can solve the problems of inaccurate dosage, limitation, and technical difficulty of semi-solid preparations, and improve bioavailability degree, reduce the frequency of dosing, and enhance the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
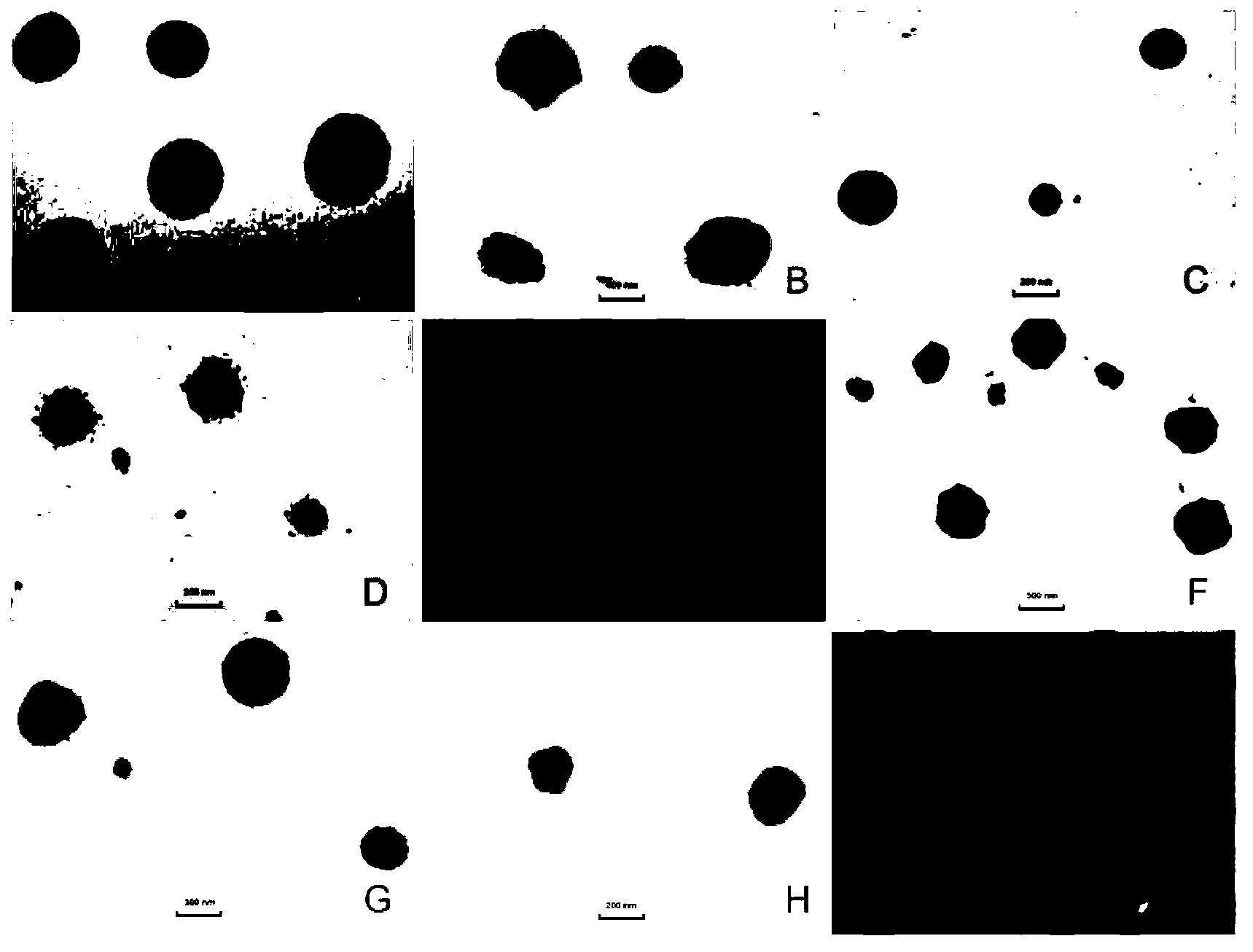
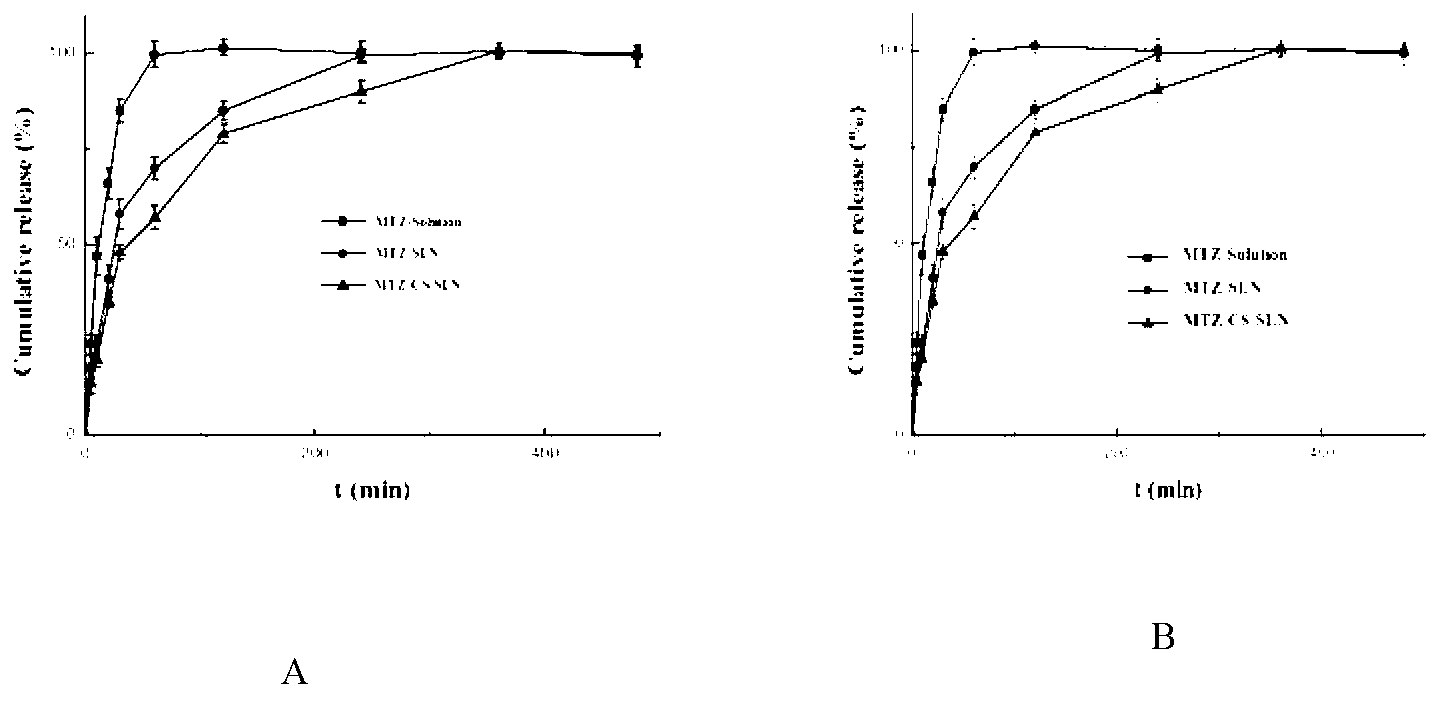
[0027]Take 5 mg of methazolamide, 100 mg of glyceryl monostearate (GMS), and 75 mg of phospholipid, add 5 mL of absolute ethanol, and heat to dissolve at 70 ° C to form an organic phase. Another 15 mL of a solution containing 150 mg Tween80 and 150 mg PEG400 was taken to form the inner aqueous phase. Aspirate the organic phase with a glass syringe through 5 # Slowly drop the organic phase into the internal water phase at the same temperature under stirring at 1200rpm, and continue stirring to completely evaporate the organic solvent to obtain colostrum (about 5mL); under stirring at 1000rpm, quickly pour the obtained colostrum into In the continuous phase (0-4°C) of 5 times the volume of colostrum, the continuous phase is a pH 4 acetic acid solution containing 5% (w / v) mannitol and 2.5 mg / mL chitosan. Stirring continued to solidify for 2 hours, returned to room temperature, and passed through a 0.45 μm microporous membrane to obtain chitosan-modified solid lipid nanoparticles...
Embodiment 2
[0029] Take 5 mg of methazolamide, 150 mg of glyceryl monostearate (GMS), and 150 mg of phospholipid, add 5 mL of absolute ethanol, and heat to dissolve at 70 ° C to form an organic phase. Another 15 mL of a solution containing 250 mg Tween80 and 250 mg PEG400 was taken to form the inner aqueous phase. Aspirate the organic phase with a glass syringe through 5 # Slowly drop the organic phase into the internal water phase at the same temperature under stirring at 1200rpm, and continue stirring to completely evaporate the organic solvent to obtain colostrum (about 5mL); under stirring at 1000rpm, quickly pour the obtained colostrum into In the continuous phase (0-4°C) of 10 times the volume of colostrum, the continuous phase is a pH 4 acetic acid solution containing 5% (w / v) mannitol and 2 mg / mL chitosan. Stirring continued to solidify for 2 hours, returned to room temperature, and passed through a 0.45 μm microporous membrane to obtain chitosan-modified solid lipid nanoparticle...
Embodiment 3
[0031] Take 5 mg of methazolamide, 25 mg of glyceryl monostearate (GMS), and 25 mg of phospholipids, add 5 mL of absolute ethanol, and heat to dissolve at 70 ° C to form an organic phase. Another 15 mL of a solution containing 50 mg Tween80 and 50 mg PEG400 was taken to form the inner aqueous phase. Aspirate the organic phase with a glass syringe through 5 # Slowly drop the organic phase into the internal water phase at the same temperature under stirring at 1200rpm, and continue stirring to completely evaporate the organic solvent to obtain colostrum (about 5mL); under stirring at 1000rpm, quickly pour the obtained colostrum into In the continuous phase (0-4°C) of 5 times the volume of colostrum, the continuous phase is a pH 6 acetic acid solution containing 5% (w / v) mannitol and 0.2 mg / mL chitosan. Stirring continued to solidify for 2 hours, returned to room temperature, and passed through a 0.45 μm microporous membrane to obtain chitosan-modified solid lipid nanoparticles....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| viscosity average molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com