Helicene analogs and preparation method thereof
A technology of helicene and compound, applied in the field of helicene analogs and their preparation, can solve the problems of small type and quantity, and achieve the effects of simple method, easily available raw materials, and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention also provides a preparation method of helicene analogs, comprising the following steps:
[0039] Refluxing solid phosgene and bisimine compound in anhydrous tetrahydrofuran under the action of low-valent titanium reagent;
[0040] The product obtained by the reflux reaction is mixed with hydrochloric acid, then extracted, washed with water, and dried to obtain the helicene analogue.
[0041] The solid phosgene chemical name described in the present invention is two (trichloromethyl) carbonates, and structure is as shown in formula (V),
[0042]
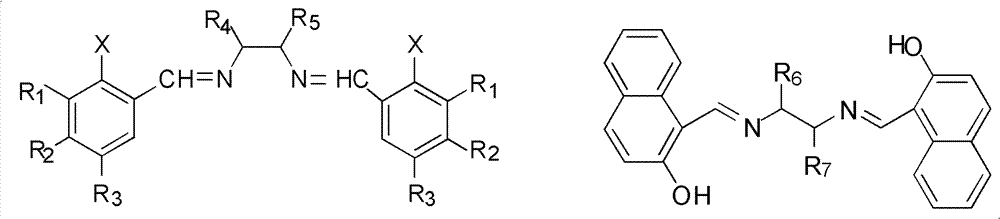
[0043] The bisimine compound has a structure shown in formula (III) or formula (IV),
[0044]
[0045] Formula (III), Formula (IV),
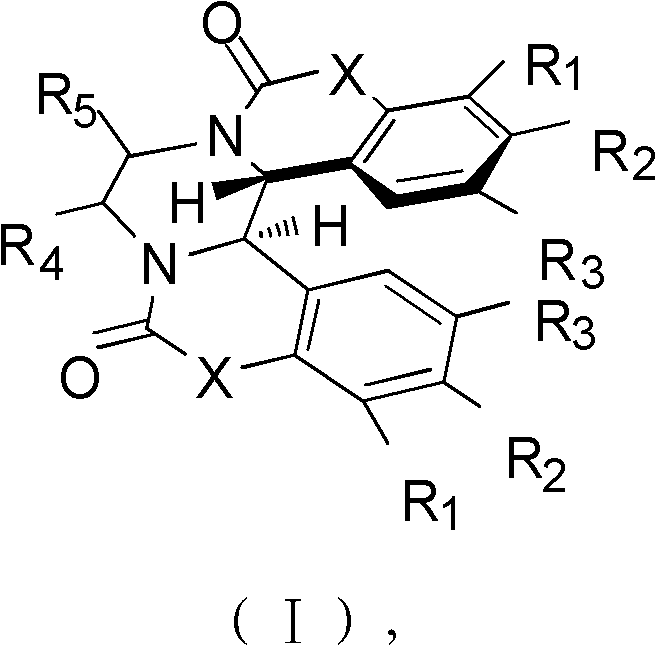
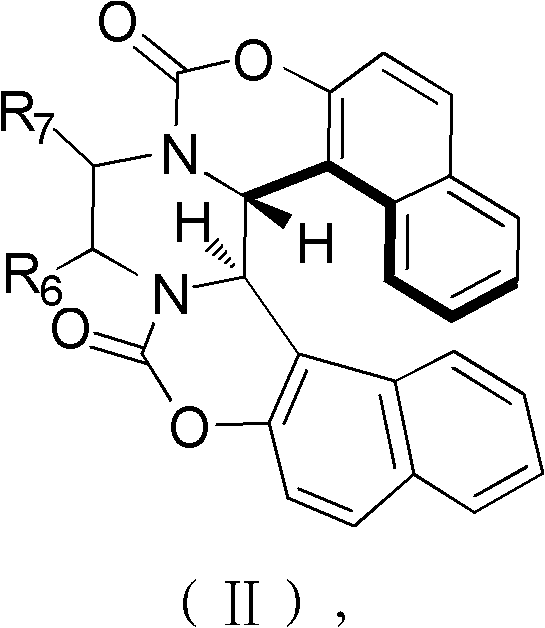
[0046] Among them, R 1 H, OCH 3 , Cl or Br; R 2 H, OCH 3 , Cl, Br or OCH 2 O; R 3 H, OCH 3 , Cl, Br or OCH 2 O; R 4 for H, CH 3 or cyclohexyl; R 5 Is H or cyclohexyl; X is OH or NO 2 ; 6 and R 7 are each independently H or cyclohexyl.
[0047] Accordin...
Embodiment 1
[0058] Add samarium powder (0.9g, 6mmol) and 10mL of anhydrous THF into a 100mL three-necked flask, slowly inject TiCl under nitrogen protection, room temperature and stirring 4 (0.66mL, 6mmol) to obtain the mixture, reflux for 2h, and cool to room temperature to obtain a low-valent titanium reagent in black paste;
[0059] At room temperature and under the protection of nitrogen, the o-hydroxybisimine (1mmol) of the structure of formula (III) and solid phosgene (2mmol) were dissolved in 10mL THF, wherein, X is OH, R 1 for H, R 2 for OCH 3 , R 3 for H, R 4 for H, R 5 H, to obtain a mixed solution, slowly drop the mixed solution into the low-valent titanium reagent, add, reflux for 2 hours, after the reaction, mix with 100mL of 3% hydrochloric acid, and extract with 50mL of chloroform for 3 Once, combine the organic layers, wash 3 times with 50mL water until neutral, dry the organic layer with anhydrous sodium sulfate, filter off the desiccant, evaporate the solvent under ...
Embodiment 2
[0062] With the o-hydroxyl bisimine (1mmol) and solid phosgene (2mmol) of formula (III) structure as raw materials, wherein, X is OH, R 1 for H, R 2 for H, R 3 for H, R 4 for H, R 5 For H, the same preparation method as in Example 1 was used to prepare 5,10-dioxa-6,9-dioxo-18,20-diazadecahydro[5]helicene with a yield of 88 %.
[0063] The NMR data of the 5,10-dioxa-6,9-dioxo-18,20-diazadecahydro[5]helicene prepared in this example are: 1 H NMR (400Hz, DMSO-d6 )δ: 7.39(t, J=7.6Hz, 2H, ArH), 7.14-7.16(m, 2H, ArH), 6.95(t, J=7.6Hz, 2H, ArH), 6.19-6.21(m, 2H, ArH), 5.02(s, 2H, 2×CH), 4.25-4.33(m, 2H, CH 2 ), 3.26-3.36 (m, 2H, CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com