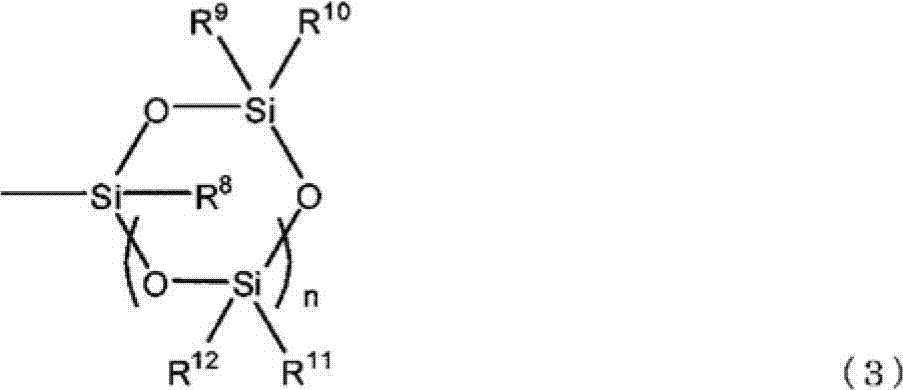
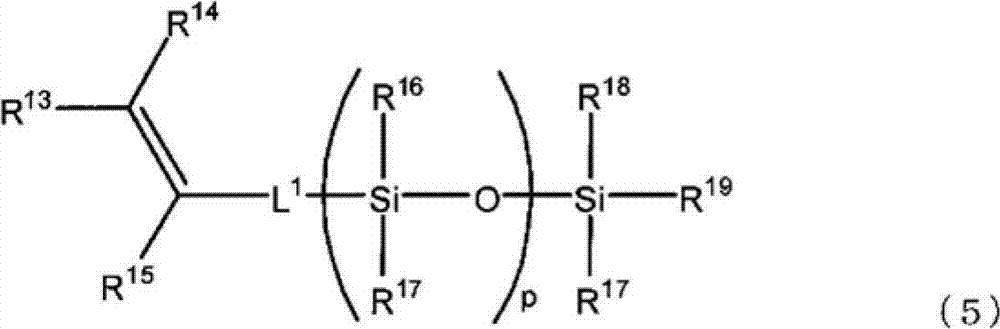
Ring-opening polymer of cyclopentene and method for producing same
A manufacturing method and technology of cyclopentene, applied in tire parts, transportation and packaging, special tires, etc., can solve the problems of low heat generation, low Mooney viscosity, poor rubber properties, etc., and achieve excellent rubber properties and low heat generation Excellent properties and excellent Mooney viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Add 1.0 wt% WCl to a glass container with a stirrer under nitrogen atmosphere 6 8.7 parts / toluene solution and 4.3 parts of 2.5 weight% diisobutylmono(n-hexyloxide)aluminum / toluene solution prepared in Reference Example 1 were stirred for 15 minutes to obtain a catalyst solution. In addition, under a nitrogen atmosphere, add 150 parts of cyclopentene and 0.22 parts of allyltriethoxysilane into a pressure-resistant glass reaction vessel with a stirrer, and add 13 parts of the catalyst solution prepared above to it, and put it at 25 ° C Polymerization was carried out for 6 hours. After 6 hours of polymerization, add excess isopropanol to the pressure-resistant glass reaction vessel to stop the polymerization, and then inject the solution in the pressure-resistant glass reaction vessel into the solution containing 2,6-di-tert-butyl-p-cresol (BHT) in an apparent excess of isopropanol. Next, the precipitated polymer was recovered, washed with isopropanol, and vacuum-dried ...
Embodiment 2
[0159] 30 parts of cyclopentene ring-opening polymers obtained in the same manner as in Example 1 were dissolved in 150 parts of tetrahydrofuran, 2.5 parts of 1 equivalent aqueous hydrochloric acid were added, and stirred at 80° C. for 4 hours to carry out the hydrolysis reaction ( A hydrolysis reaction in which a triethoxysilyl group is reacted to obtain a trihydroxysilyl group). After the hydrolysis reaction is complete, a significant excess of isopropanol containing 2,6-di-tert-butyl-p-cresol (BHT) is injected. Next, the precipitated polymer was recovered, washed with isopropanol, and vacuum-dried at 40° C. for 3 days to obtain 30 parts of a cyclopentene ring-opened polymer. Wherein, carried out for the gained cyclopentene ring-opening polymer 1 In H-NMR measurement, the following results were confirmed: the peak derived from the ethyl group of the triethoxysilyl group almost disappeared, and more than 99% of the triethoxysilyl group was hydrolyzed to be converted into a t...
Embodiment 3
[0161] Add 1.0 wt% WCl to a glass container with a stirrer under nitrogen atmosphere 6 8.7 parts / toluene solution and 4.3 parts of 2.5% by weight diisobutyl mono(n-butoxide)aluminum / toluene solution prepared in Reference Example 2, after stirring for 10 minutes, add 0.039 parts of ethyl acetate and carry out 10 minutes By stirring, a catalyst solution was obtained. In addition, under a nitrogen atmosphere, 150 parts of cyclopentene and 0.29 parts of 2-styrylethyltrimethoxysilane were added to a pressure-resistant glass reaction vessel with a stirrer, and 13 parts of the catalyst solution prepared above were added thereto, Polymerization was carried out at 25°C for 6 hours. After 6 hours of polymerization, add excess isopropanol to the pressure-resistant glass reaction vessel to stop the polymerization, and then inject the solution in the pressure-resistant glass reaction vessel into the solution containing 2,6-di-tert-butyl p-cresol (BHT) in a large excess of isopropanol. N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com