Preparation method for nano-sliver/cellulose nanocrystalline composite particle
A nanocrystalline composite and cellulose technology, applied in the direction of nanotechnology, can solve the problems of weakening the reinforcement effect of biodegradable polymer matrix materials, destroying high-strength and high-mode characteristics, and not being able to become a reduction point, so as to shorten the preparation cycle and chemical Fewer drugs, improve the effect of combination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
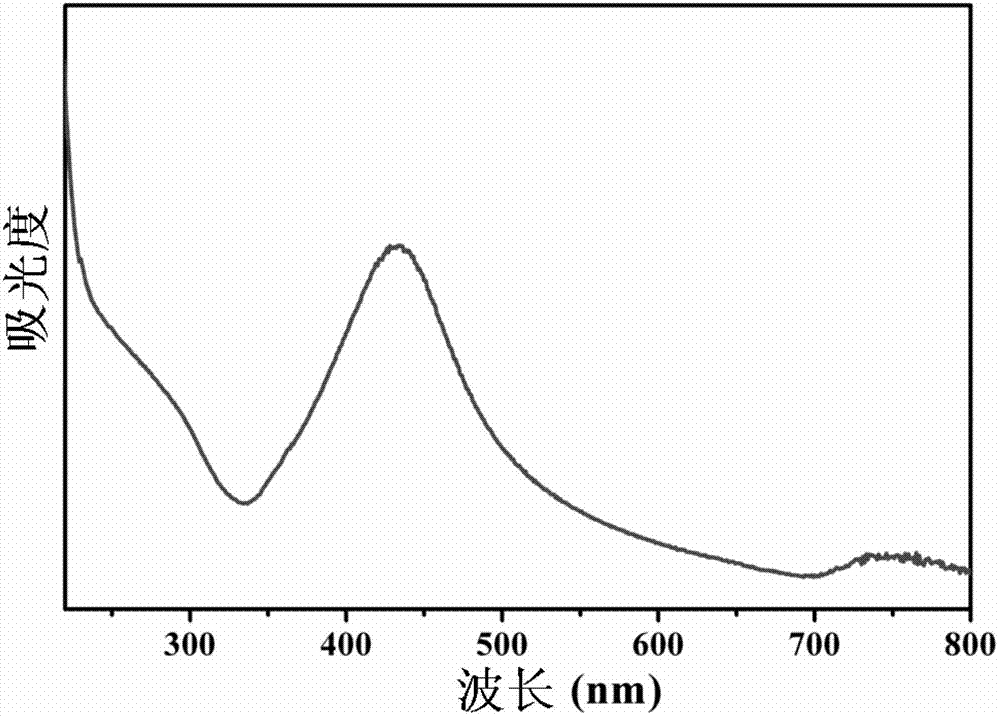
[0025] Add microcrystalline cellulose to the formic acid / sulfuric acid solution, wherein the solid-to-liquid ratio of microcrystalline cellulose to the mixed acid solution is 1:30g / mL, and the volume ratio of formic acid (5mol / L) to sulfuric acid (8mol / L) in the mixed acid solution 8:1, the mixed solution was reacted at 85°C for 10h, and after the reaction was completed, the reaction product was washed with deionized water until it was neutral, and the formylated cellulose nanocrystal (CNC) could be obtained; the formylated cellulose nanocrystal Add crystal liquid to 0.01mol / L silver ammonia solution, the solid-to-liquid ratio of aldylated CNC to silver ammonia solution is 1:100g / mL, the mixed solution is reacted at 85°C for 15min, after natural cooling, dilute with deionized water The reaction product is centrifuged several times to remove inorganic ions, and then the dispersion is freeze-dried to obtain a nano-silver / CNC composite material. After the material is tested by a f...
Embodiment 2
[0027] Add the cellulose fiber to the formic acid / oxalic acid solution, wherein the solid-liquid ratio of the cellulose fiber to the mixed acid solution is 1:80g / mL, and the volume ratio of formic acid (3mol / L) to oxalic acid (5mol / L) in the mixed acid solution is 7 : 1, the mixed solution was reacted at 90°C for 18h, and after the reaction was completed, the reaction product was washed with deionized water until it was neutralized, and the formylated cellulose nanocrystal (CNC) could be obtained; the formylated cellulose nanocrystal liquid Add 0.5mol / L silver-ammonia solution, the solid-liquid ratio of aldehylated CNC to silver-ammonia solution is 1:50g / mL, and the mixed solution is reacted at 70°C for 45min. After natural cooling, dilute the reaction product with deionized water , several times of centrifugation to remove inorganic ions, and then vacuum-dry the dispersion to obtain nano-silver / CNC composite material. After the material is tested by field emission scanning ele...
Embodiment 3
[0029]Add cotton to the formic acid / nitric acid solution, wherein the solid-liquid ratio of cotton to the mixed acid solution is 1:60g / mL, and the volume ratio of formic acid (8mol / L) to nitric acid (3mol / L) in the mixed acid solution is 9:1, mix The solution was reacted at 75°C for 15 hours. After the reaction was completed, the reaction product was washed with deionized water until it was neutral, and aldehyde-based cellulose nanocrystals (CNC) could be obtained; add 0.09mol / In the silver-ammonia solution of L, the solid-to-liquid ratio of the aldylated CNC to the silver-ammonia solution is 1:300g / mL, and the mixed solution is reacted at 72°C for 30min. After natural cooling, the reaction product is diluted with deionized water and centrifuged several times. Remove the inorganic ions, and then freeze-dry the dispersion to obtain the nano-silver / CNC composite material. After the material is tested by the field emission scanning electron microscope, the obtained aldehyde-forme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com