Sedative and soporific compound, and preparation method and application thereof
A compound, phenyl technology, applied in the field of sedative and hypnotic compounds, can solve the problems restricting the clinical application of drugs, achieve the effect of inhibiting the number of spontaneous activities, simple process route, obvious social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
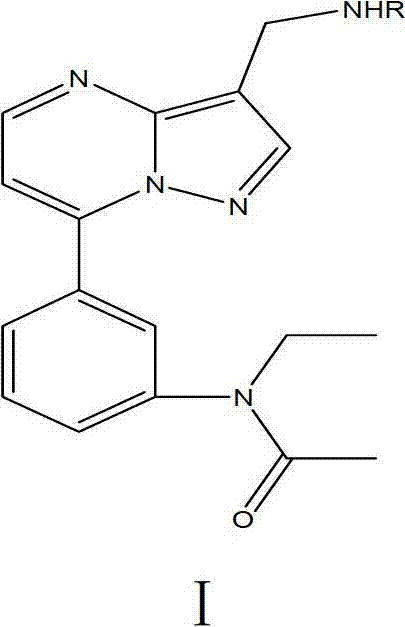
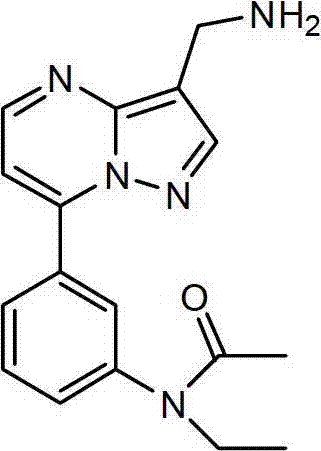
[0050] Example 1 Preparation of N-ethyl-N-3-[7-aminomethylpyrazolo[1,5a]pyrimidinylphenyl]acetamide (2 for short)
[0051]
[0052] The processing steps of the present embodiment are as follows:
[0053] In a 100mL pear-shaped flask, add 20mmol of ferric chloride hexahydrate in 10mL of aqueous solution and 10mmol of N-ethyl-N-3-[7-(3-cyanopyrazolo[1,5a]pyrimidinyl)phenyl ] The mixed liquid of DMF solution of acetamide was cooled with ice water at 00C, and 0.1mol sodium borohydride was added several times under stirring. After the addition was completed, it was reacted at room temperature for 5 hours, and the reaction was tracked by TLC. After the end, filter, extract with EA several times, wash with water several times, and the organic layer is washed with anhydrous Na 2 SO 4 Dry and spin dry, and the crude product is purified by column chromatography to obtain N-ethyl-N-3-[7-aminomethylpyrazolo[1,5a]pyrimidinylphenyl]acetamide as a white powdery solid , yield 58%, m.p.1...
Embodiment 2
[0056] Example 2: N-[7-(N-acetyl-N-ethylamino-3-)phenyl[pyrazolo[1,5a]pyrimidinyl]-3-]methylbenzenesulfonamide (abbreviated as 4a ) preparation
[0057]
[0058] The process steps of this embodiment are as follows: in a 25mL pear-shaped bottle, add 10mL THF, add 0.3mmol NaH under ice bath conditions, stir for 5 minutes, add 0.1mmol N-ethyl-N-3-[7-aminomethyl Pyrazolo[1,5a]pyrimidinylphenyl]acetamide, continue to add 0.12mmol benzenesulfonyl chloride after dissolving, react at room temperature for 1h after the addition, follow the reaction by TLC, extract with EA, wash with water, and use Anhydrous Na 2 SO 4 Drying and spin-drying, the crude product was purified by column chromatography to obtain N-[7-(N-acetyl-N-ethylamino-3-)phenyl[pyrazolo[1,5a]pyrimidinyl]-3 -] Toluenesulfonamide, white powdery solid, 92% yield, m.p.160-161°C. 1 H NMR (400MHz, CDCl 3 )δ(ppm):0.98(t,J=7.2Hz,3H),1.66(s,3H),1.89(m,1H),2.25(m,1H),3.59(q,J=7.2Hz,2H) ,3.74(m,1H),3.88(m,1H),5.26(t,J=6.8Hz...
Embodiment 3
[0059] Embodiment 3: N-[7-(N-acetyl-N-ethylamino-3-)phenyl[pyrazolo[1,5a]pyrimidinyl]-3-]methyl-p-bromobenzenesulfonamide ( Abbreviated 4b) Preparation
[0060]
[0061] The processing steps of the present embodiment are as follows:
[0062] The process steps of this embodiment are as follows: in a 25mL pear-shaped bottle, add 10mL THF, add 0.3mmol NaH under ice bath conditions, stir for 5 minutes, add 0.1mmol N-ethyl-N-3-[7-aminomethyl Pyrazolo[1,5a]pyrimidinylphenyl]acetamide, continue to add 0.12mmol benzenesulfonyl chloride after dissolving, react at room temperature for 1h after the addition, follow the reaction by TLC, extract with EA, wash with water, and use Anhydrous Na 2 SO 4 Drying and spin-drying, the crude product was purified by column chromatography to obtain N-[7-(N-acetyl-N-ethylamino-3-)phenyl[pyrazolo[1,5a]pyrimidinyl]-3 -] Methyl-p-bromobenzenesulfonamide, white powdery solid yield 89%, m.p.137-139°C. 1 H NMR (400MHz, CDCl 3 )δ(ppm):1.08(t,J=7.2Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com