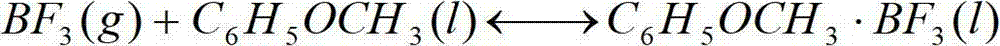Method and device for separately producing enriched boron-10 (10B) by using multiple serial towers
A technology of enrichment and multiple towers, applied in the field of separation, can solve the problems of difficult industrial production, many sub-processes, and long process flow, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] Example 1: The boron trifluoride raw material gas (1) enters the complexation tower from the bottom, and forms a 1:1 boron trifluoride·anisole ether compound with the excess anisole flowing down from the top of the tower. The complexation reaction of boron trifluoride and anisole complexing agent is an exothermic reversible reaction, and the reaction will naturally proceed in the direction of complexation when the temperature is lowered to 10-15°C. When the complex is heated to 140-150°C, it will be decomposed into boron trifluoride and anisole complexing agent. In the chemical exchange system at 15-20°C, the cracked boron trifluoride gas rises from the bottom of the exchange tower, and contacts with the boron trifluoride-anisole complex descending from the top of the tower in countercurrent, and an exchange reaction occurs. Reciprocate, and finally get more than 95% enrichment at the bottom of the exchange tower 10 Product B.
example 2
[0025]Example 2: The boron trifluoride raw material gas enters the complexation tower from the bottom, and forms a 1:1 boron trifluoride·anisole ether compound with the excess anisole flowing down from the top of the tower. The complexation reaction between boron trifluoride and anisole complexing agent is an exothermic reversible reaction. When the temperature is lowered to 15-20°C, the reaction will naturally proceed in the direction of complexation. When the complex is heated to 150-160°C, it will be decomposed into boron trifluoride and anisole complexing agent. In the chemical exchange system at 20-25°C, the cracked boron trifluoride gas rises from the bottom of the exchange tower, and contacts with the boron trifluoride-anisole complex descending from the top of the tower in countercurrent, and an exchange reaction occurs. Reciprocate, and finally get more than 95% enrichment at the bottom of the exchange tower 10 Product B.
example 3
[0026] Example 3: The boron trifluoride raw material gas enters the complexing tower from the bottom, and forms a 1:1 boron trifluoride·anisole ether compound with the excess anisole flowing down from the top of the tower. The complexation reaction of boron trifluoride and anisole complexing agent is an exothermic reversible reaction, and the temperature is lowered to 20-25°C, and the reaction naturally proceeds in the direction of complexation. When the complex is heated to 160-170°C, it will be decomposed into boron trifluoride and anisole complexing agent. In the chemical exchange system at 25-30°C, the cracked boron trifluoride gas rises from the bottom of the exchange tower, and contacts with the boron trifluoride-anisole complex descending from the top of the tower in countercurrent, and an exchange reaction occurs. Reciprocate, and finally get more than 95% enrichment at the bottom of the exchange tower 10 Product B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com