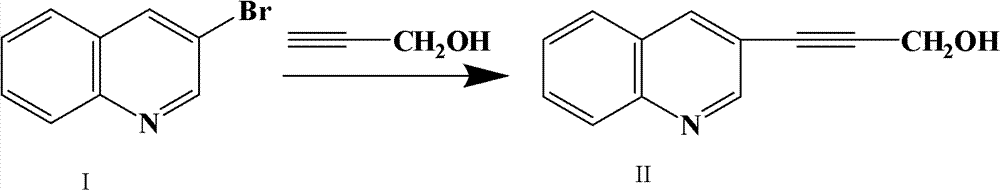
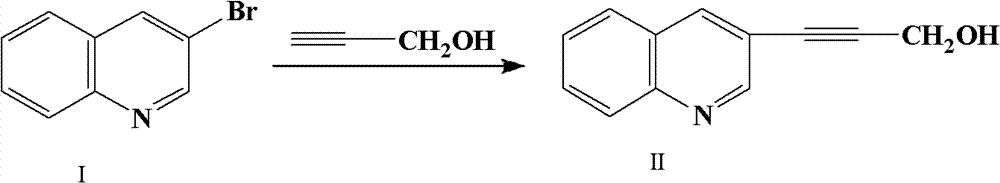
Preparation method of 3-(3-quinolyl)-2-propyne-1-alcohol
A quinolinyl and propyne technology is applied in the field of preparation of 3--2-propyn-1-ol, can solve problems such as non-compliance with environmental protection requirements, unfriendly environment, etc., and achieves cost reduction, reaction yield improvement, The effect of reducing harm to the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of 3-(3-quinolyl)-2-propyn-1-alcohol:
[0021] In the 100ml reaction flask, add 2g 3-bromoquinoline, 0.8g propargyl alcohol, 0.04g cuprous iodide, 0.07g bis(triphenylphosphine) palladium dichloride (Pd(PPh 3 ) 2 Cl 2 ), 2.8g of potassium carbonate, then add 40ml of water, heat to reflux, and the reaction of raw materials is complete after 40min. Pour the reaction solution into a separatory funnel, extract with 60ml of ethyl acetate, wash the organic layer with 50ml of saturated sodium bicarbonate and 50ml of saturated sodium chloride successively, dry with anhydrous sodium sulfate, filter with suction, and evaporate the filtrate to dryness Yield 1.74 g (98.8%) of solid.
Embodiment 2
[0023] In the 100ml reaction flask, add 2g 3-bromoquinoline, 0.8g propargyl alcohol, 0.4g cuprous iodide, 0.7gPd(PPh 3 ) 2 Cl 2 , 2.8g of potassium carbonate, then add 40ml of water, heat to reflux, 40min after the reaction of raw materials is complete. Pour the reaction solution into a separatory funnel, extract with 60ml of ethyl acetate, wash the organic layer with 50ml of saturated sodium bicarbonate and 50ml of saturated sodium chloride successively, dry with anhydrous sodium sulfate, filter with suction, and evaporate the filtrate to dryness Yield 1.75 g (99.4%) of solid.
Embodiment 3
[0025] In the 100ml reaction flask, add 2g 3-bromoquinoline, 0.8g propargyl alcohol, 0.08g cuprous iodide, 0.07gPd(PPh 3 ) 2 Cl 2 , 2.8g of potassium carbonate, then add 40ml of water, heat to reflux, 40min after the reaction of raw materials is complete. Pour the reaction solution into a separatory funnel, extract with 60ml of ethyl acetate, wash the organic layer with 50ml of saturated sodium bicarbonate and 50ml of saturated sodium chloride successively, dry with anhydrous sodium sulfate, filter with suction, and evaporate the filtrate to dryness Yield 1.73 g (98.3%) of solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com