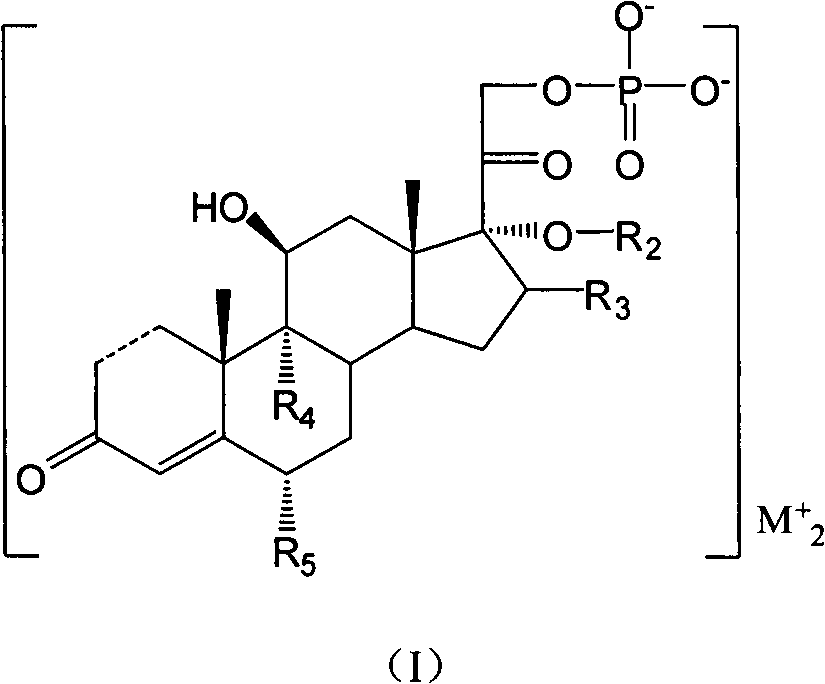
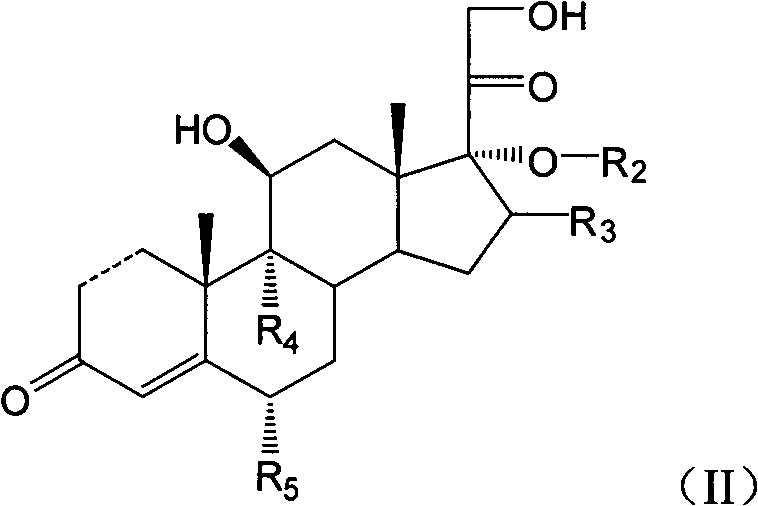
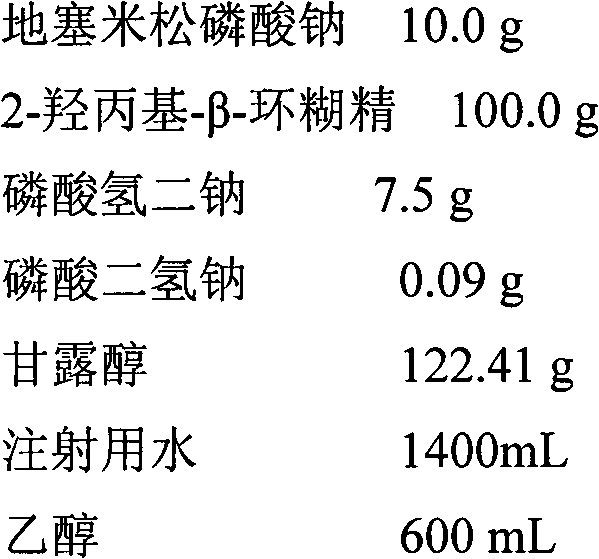
Cortical hormone alkali metal phosphate composition
A composition and compound technology, which is applied in the direction of non-active ingredients of polymer compounds, medical preparations of non-active ingredients, powder delivery, etc., and can solve the problems of prone to spray bottles, high levels of related substances, and inability to prepare freeze-dried powder injections, etc. problem, achieve the effect of improving content stability and simplifying production process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2-1
[0061]
[0062] (1) Dissolve cyclodextrin in 20% prescription water for injection and add dexamethasone sodium phosphate to dissolve
[0063] (2) Dissolve phosphate in 80% prescription water for injection, add mannitol and make it dissolve
[0064] (3) Combine the above 2 solutions and mix well
[0065] (4) Add the prescribed amount of ethanol to the above solution and mix evenly, then filter and sterilize with a 0.22 μm filter element, and spray dry to obtain the dexamethasone sodium phosphate composition.
[0066] The spray drying conditions are as follows:
[0067] Hot air flow: 0.80m 3 / min
[0068] Inlet temperature: 100°C
[0069] Outlet temperature: 65°C
[0070] Spray pressure: 120kpa
[0071] Liquid flow rate: 5mL / min
[0072] (5) the dexamethasone sodium phosphate composition obtained in step (4) is subpackaged into powder injection, specification 5mg / 120.0mg (dexamethasone sodium phosphate / gross weight)
Embodiment 2-2
[0074]
[0075] (1) Dissolve phosphate in 20% prescription water for injection and add dexamethasone sodium phosphate to dissolve
[0076] (2) Dissolve mannitol in the remaining 80% water for injection
[0077] (3) Combine the above 2 solutions and mix well
[0078] (4) Add the prescribed amount of ethanol to the above solution and mix well, then pass the medicinal solution through a 0.22 μm filter element to filter and sterilize. Spray drying is carried out to obtain a dexamethasone sodium phosphate composition. Spray condition is the same as embodiment 2-1
[0079] (5) the dexamethasone sodium phosphate composition obtained in step (4) is subpackaged into powder injection, specification 5mg / 70.0mg (dexamethasone sodium phosphate / gross weight)
Embodiment 3-1
[0081]
[0082] (1) Dissolve cyclodextrin in half the prescription amount of water for injection and add betamethasone sodium phosphate to dissolve
[0083] (2) Dissolve phosphate in half the prescribed amount of water for injection, add mannitol and dissolve it
[0084] (3) Combine the above 2 solutions and mix well
[0085] (4) Add the prescribed amount of ethanol to the above solution, filter and sterilize through a 0.22 μm filter element, mix evenly, and spray dry to obtain the betamethasone sodium phosphate composition.
[0086] The spray drying conditions are as follows:
[0087] Hot air flow: 1.05m 3 / min
[0088] Inlet temperature: 100°C
[0089] Outlet temperature: 85°C
[0090] Spray pressure: 120kpa
[0091] Liquid flow rate: 8mL / min
[0092] (5) The betamethasone sodium phosphate composition obtained in step (4) is divided into powder injections, the specification is 5 mg / 110.0 mg (betamethasone sodium phosphate / total weight).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com